by Josep Carreras Leukaemia Research Institute
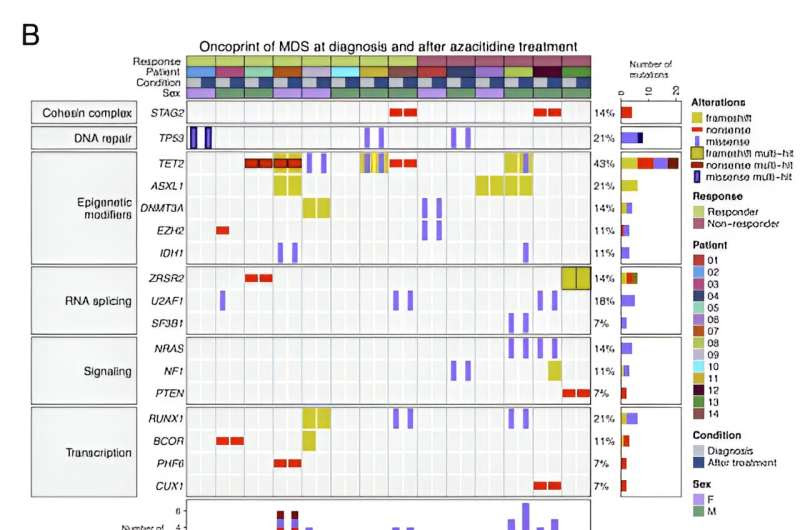
Credit: Cancer Research Communications (2024). DOI: 10.1158/2767-9764.CRC-23-0389
Alterations in the chemical modifications that control gene expression, known as epigenetics, have proven to be one of the most characteristic properties of all human tumors. This realization has generated the development of intense pharmacological research to find drugs that act at this level against cancer. Today there are nine epigenetic drugs approved for use in oncology, especially in leukemias, lymphomas and soft tissue tumors. However, a mystery remains: Why do some patients respond clinically to these compounds others show resistance to their action?
A study led by Dr. Manel Esteller, Director of the Josep Carreras Leukaemia Research Institute, ICREA Research Professor and Chairman of Genetics at the School of Medicine of the University of Barcelona and appearing in the journal Cancer Research Communications, provides the first answer to this question by showing how the persistence of certain single cancer cells with mutations is associated with the lack of clinical benefit from these pharmacological principles.
“We decided to focus our study on a type of bone marrow cancer where leukemia blood cells are formed, called myelodysplastic syndrome (MDS), because the treatment of choice is an epigenetic drug called azacitidine, an inhibitor of DNA methylation. We studied what was happening at the DNA and protein levels in thousands of cells separated from these patients at two time points: before and after receiving the epigenetic therapy,” explains Dr. Esteller.
“We managed to characterize more than 30 cell subtypes and 50 genes, observing that the patients where pharmacological treatment had an effect had a particular profile at the individual cell level: they presented a decrease in the number of mutations in stem and progenitor cells and in immature granulocytes and monocytes. This suggests that if we do not eliminate these altered primitive cells, which appear early during the tumor process, the therapy has little chance of success.
“The good news for patients resistant to epigenetic drugs is that we have detected that some of the new mutations that appear may now be targets of other drugs specifically directed against them. As if it were a game of cat and mouse between the doctor and the cancer, the tumor’s strength against one drug generates its vulnerability against another drug. Hence the importance of molecular studies at the single cell level that allow us to predict not only the prognosis of the disease, but also what this leukemia may be sensitive to,” concludes the researcher.
More information: Ignacio Campillo-Marcos et al, Single-Cell Multiomics Analysis of Myelodysplastic Syndromes and Clinical Response to Hypomethylating Therapy, Cancer Research Communications (2024). DOI: 10.1158/2767-9764.CRC-23-0389
Provided by Josep Carreras Leukaemia Research Institute

Leave a Reply