by Karl Linden, The Conversation
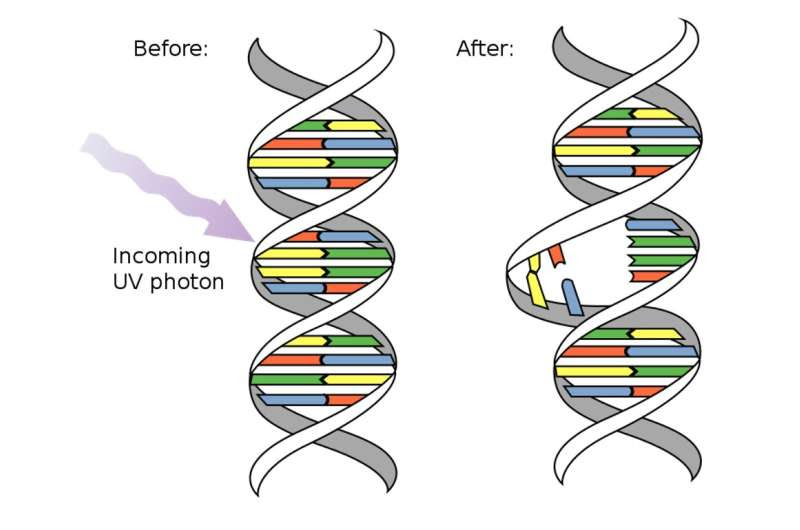
When UV light enters a cell, it breaks the bonds that hold DNA or RNA together. Credit: NASA/David Herring via WikimediaCommons
Scientists have long known that ultraviolet light can kill pathogens on surfaces and in air and water. UV robots are used to disinfect empty hospital rooms, buses and trains; UV bulbs in HVAC systems eliminate pathogens in building air; and UV lamps kill bugs in drinking water.
Perhaps you have seen UV wands, UV LEDs and UV air purifiers advertised as silver bullets to protect against the coronavirus. While decades of research have looked at the ability of UV light to kill many pathogens, there are no set standards for UV disinfection products with regard to the coronavirus. These products may work to kill SARS-CoV-2, the virus that causes COVID-19, but they also may not.
I am an environmental engineer and expert in UV disinfection. In May 2021, my colleagues and I set out to accurately test various UV systems and see which was the most effective at killing off—or inactivating—SARS-CoV-2.
How does UV light kill a virus?
Light is categorized by wavelength—the distance between peaks of a wave of light—and is measured in nanometers. UV wavelengths range from 100 to 400 nanometers—shorter in wavelength than the violet hues in visible light—and are invisible to the human eye. As wavelength shortens, photons of light contain higher amounts of energy.
Different wavelengths of UV light work better than others for inactivating viruses, and this depends on how well the wavelengths are absorbed by the virus’s DNA or RNA. When UV light gets absorbed, the photons of light transfer their energy to and damage the chemical bonds of the genetic material. The virus is then unable to replicate or cause an infection. Researchers have also shown the proteins that viruses use to attach to a host cell and initiate infection—like the spike proteins on a coronavirus—are also vulnerable to UV light.
The dose of light matters too. Light can vary in intensity—bright light is more intense, and there is more energy in it than in dim light. Being exposed to a bright light for a short time can produce the same UV dose as being exposed to a dim light for a longer period. You need to know the right dose that can kill coronavirus particles at each UV wavelength.
Making ultraviolet lights safe for people
Traditional UV systems use wavelengths at or around 254 nanometers. At these wavelengths the light is dangerous to human skin and eyes, even at low doses. Sunlight includes UV light near these wavelengths; anyone who has ever gotten a bad sunburn knows just how dangerous UV light can be.
However, recent research has shown that at certain UV wavelengths—specifically below 230 nanometers—the high-energy photons are absorbed by the top layers of dead skin cells and don’t penetrate into the active skin layers where damage can occur. Similarly, the tear layer around eyes also blocks out these germicidal UV rays.
This means that at wavelengths of UV light below 230 nanometers, people can move around more freely while the air around them is being disinfected in real time.
Researchers used this setup to test multiple different UV lights at various doses to see what it took to kill SARS-CoV-2. Credit: Karl Linden, CC BY-ND
Testing different wavelengths
My colleagues and I tested five commonly used UV wavelengths to see which work best to inactivate SARS-CoV-2. Specifically, we tested how large a dose is needed to kill 90% to 99.9% of the viral particles present.
We ran these tests in a biosafety level three facility at the University of Arizona that is built to handle lethal pathogens. There we tested numerous lights across the UV spectrum, including UV LEDs that emit light at 270 and 282 nanometers, traditional UV tube lamps at 254 nanometers and a newer technology called an excited dimer, or excimer, UV source at 222 nanometers.
To test each device we spiked a sample of water with millions of SARS-CoV-2 viruses and coated a petri dish with a thin layer of this mixture. We then shined UV light on the petri dish until we achieved a specific dose. Finally we examined the viral particles to see if they could still infect human cells in culture. If the viruses could infect the cells, the dose was not high enough. If the viruses did not cause an infection, the UV source at that dose had successfully killed the pathogen. We carefully repeated this process for a range of UV doses using the five different UV devices.
While all of the wavelengths we tested can inactivate SARS-CoV-2 at very low doses, the ones that required the lowest dose were the systems that emit UV light at a wavelength of 222 nanometers. In our experiment, it took a dose of less than 2 millijoules of energy per square centimeter to kill 99.9% of viral particles. This translates to needing about 20 seconds to disinfect a space receiving a low intensity of short wavelength UV light, similar to that used in our test.
These 222-nanometer systems are almost twice as effective as conventional UV tube lamps, which are often used in ultraviolet disinfecting systems. But importantly, the winning lamp also happens to be the safest for humans, too. At the same UV light intensity it takes to kill 99.9% of SARS-CoV-2 in 20 seconds, a person could be safely exposed to 222-nanometer light for up to one hour and 20 minutes.
What this means is that widely available types of UV lamp lights can be used to safely knock down levels of the coronavirus with people present.
Better use of existing tech
Many places or organizations—ranging from the U.S. Air Force to the Space Needle in Seattleto Boeing—are already using or investigating ways to use UV light in the 222 nanometer range to protect public health.
I believe that our findings are important because they quantify the exact doses needed to achieve various levels of SARS-CoV-2 control, whether that be killing 90% or 99.9% of viral particles.
Imagine coffee shops, grocery stores, school classrooms, restaurants and concert venues now made safe by this technology. And this is not a solution for just SARS-CoV-2. These technologies could help protect human health in public spaces in future times of crisis, but also during times of relative normalcy, by reducing exposure to everyday viral and bacterial threats.

Leave a Reply