By JAMES CIRRONE FOR DAILYMAIL.COM
A whopping 135 batches of blood pressure medication have been recalled over fears that the pill capsules won’t properly dissolve when ingested by patients.
Glenmark Pharmaceuticals initiated a voluntary recall of 114 batches of 750 mg Potassium Chloride in bottles containing 100 and 500 pills, with all of the specific batch numbers and expiration dates listed in a document circulated by the FDA.
Likewise, American Health Packaging voluntarily recalled 21 batches of the same medicine on behalf of BluePoint Laboratories.
According to a company announcement, the failed dissolution of the potassium chloride extended release capsules could cause high potassium levels in the blood, also known as hyperkalemia.
Hyperkalemia can cause an ‘irregular heart beat that can lead to cardiac arrest.’

Glenmark Pharmaceuticals recently initiated a voluntary recall of 114 batches of 750 mg Potassium Chloride in bottles containing 100 and 500 pills
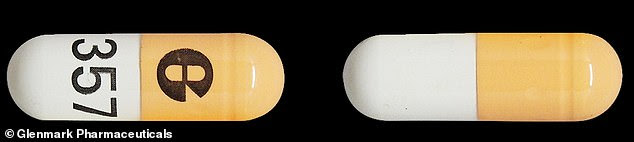
Pictured: The faulty pill capsules that have been recalled by Glenmark Pharmaceuticals and American Health Packaging

Pictured: The label found on the 500-capsule bottle of the recalled blood pressure medicine

Pictured: The label found on the 500-capsule bottle of the recalled blood pressure medicine carrying the BluePoint Laboratories brand name
Patients who take this medicine to manage high blood pressure and prevent heart or kidney failure are at extreme risk, since the bodily delivery of the pills have been compromised.
Individuals who are prescribed these particular recalled batches could experience a range of hyperkalemia symptoms, including ‘cardiac arrhythmias, severe muscle weakness, and death.’
Glenmark hasn’t yet received reports of hyperkalemia or any of the described adverse events.
These potassium chloride pills have already been distributed to wholesale, distributor and retail outlets all across the nation, leading Glenmark to write these customers letters telling them to immediately take the products of the shelves.
People who have been prescribed this medicine are being told to consult with their doctor before they stop taking it.
Additionally, the company recommends consumers call their doctor or healthcare provider if they experience the beginnings of any hyperkalemia symptoms.
The FDA is urging people to report adverse events to its MedWatch Adverse Event Reporting program either online, by regular mail or by fax.

Leave a Reply