by University of Pennsylvania
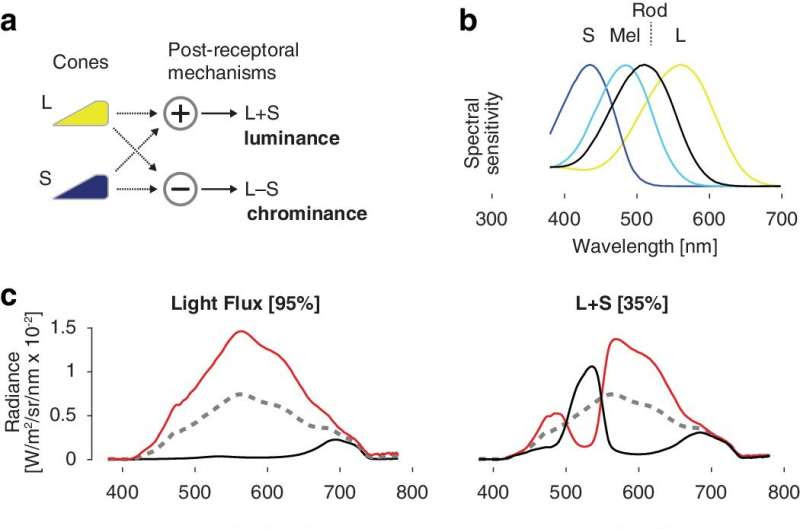
(a) Signals from the L/M (hereafter “L”) and S cones are combined and contrasted to create two postretinal channels that signal luminance and chrominance. (b) Spectral sensitivities of the canine photoreceptors. (c) Stimulus spectra used to target particular photoreceptor combinations. Each stimulus modulated between a stimulating (red) and suppressing (black) spectrum around a common background (dashed gray). The nominal contrast on the targeted photoreceptors is given in brackets. Light flux produces equal contrast on all photoreceptors. (d) A spatially uniform field of light was presented to one eye. The field modulated between a stimulating and a suppressing spectrum, following a sinusoidal temporal waveform. In measurements of pupil response the modulation frequency was 1/6 Hz, and the consensual pupil response to the stimulus was recorded from the unstimulated eye using an infrared camera. (e) In fMRI measurements, a higher frequency flicker was presented in 12-second blocks, interleaved with presentations of the static, photopic background spectrum. Credit: Translational Vision Science & Technology (2024). DOI: 10.1167/tvst.13.1.18
In the retinas of human eyes, the cones are photoreceptor cells responsible for color vision, daylight vision, and the perception of small details. As vision scientists from the Division of Experimental Retinal Therapies at the University of Pennsylvania School of Veterinary Medicine, Gustavo D. Aguirre and William A. Beltran have been working for decades to identify the basis of inherited retinal diseases. They have previously shown they could recover missing cone function by reintroducing a copy of the normal gene in photoreceptor cells.
Both humans and dogs are affected by retinal disease, and a new study of daylight vision using a canine model offers a critical insight for evaluating “whether these cell replacements—where we are introducing cones into the retinas of these dogs—is a successful approach for restoring cone vision,” says Beltran, the Corinne R. and Henry Bower Endowed Professor of Ophthalmology.
Their findings were published in Translational Vision Science & Technology. The other co-authors are Huseyin O. Taskin, a former research specialist at Penn in the GKAguirre Lab and current graduate student at the University of Toronto, and Jacqueline Wivel, a veterinary technician.
Beltran and Gustavo Aguirre teamed with researchers including cognitive neuroscientist Geoffrey K. Aguirre, a professor of neurology at the Perelman School of Medicine, bringing together knowledge on the retinal system and brain measurements. In dogs with three different kinds of naturally occurring retinal disease and in dogs with normal vision, the scientists used functional magnetic resonance imaging (fMRI) to assess brain responses to lights that stimulate only the cone cells.
The researchers found that fMRI can detect brain responses to daylight vision for black and white information as well as color information, and it can identify the area of the visual cortex that responds to stimulation of a region in the dog retina that is rich in cones and similar to the human fovea. They also found that they can use fMRI to measure the relative degree of loss of daylight vision.
Using this technique in animals with a retinal disease caused by a mutation in a gene called NPHP5, they demonstrated that gene augmentation therapy restored the response in the cortex to black and white stimulation. That makes this disease a promising one in which to study photoreceptor cell replacement as a treatment in the future.
“Canine models are useful for studying retinal diseases because they have a variety of different naturally occurring genetic disorders. The ultimate goal is to first demonstrate that these disorders can be treated in canines before translating it to human patients,” says Taskin, the first author. Gustavo Aguirre says, “The hope is that successful therapeutic approaches in people will then become available to veterinarians so that they can benefit man’s four-legged friend.”
Geoffrey Aguirre says, “The purpose of the study was to see, in different versions of these retinal diseases, how much information about daylight vision makes it to the visual system in these dogs.” This knowledge is particularly useful, he says, because figuring out whether a treatment for retinal disease has been effective requires knowing how much vision function was present prior to treatment.
Beltran says this paper shows that gene therapy can recover cone function because it looks at an animal model with no cone function and shows an improvement. He explains that in the disease caused by the NPHP5 mutation, cones are present but not functional. Animals with this disease are born day-blind but initially have some night vision, though rods—photoreceptors that allow night vision—die over a period of months, making dogs fully blind within a year.
More information: Huseyin O. Taskin et al, Cone-Driven, Geniculocortical Responses in Canine Models of Outer Retinal Disease, Translational Vision Science & Technology (2024). DOI: 10.1167/tvst.13.1.18
Provided by University of Pennsylvania

Leave a Reply