by Bob Yirka, Medical Xpress
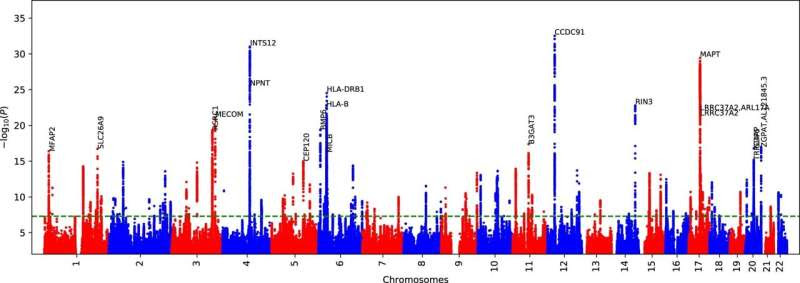
ML-based COPD GWAS Manhattan plot via DeepNull. We performed ML-based COPD GWAS where we used the same set of covariates as the Fig. 4 with one additional covariate provided by DeepNull. DeepNull model predicts the ML-based COPD using age, sex, genotype-array, and FEV1/FVC as inputs. The additional DeepNull-covariate is the DeepNull model prediction of ML-based COPD. DeepNull learns a function (that is, linear or non-linear) that predicts ML-based COPD via age, sex, genotype-array, and FEV1/FVC as inputs. Thus, this analysis is similar to the ML-based COPD GWAS conditional on FEV1/FVC where instead of assuming that FEV1/FVC has linear relationship with ML-based COPD, DeepNull handles cases where age, sex, and FEV1/FVC can have non-linear relationship with ML-based COPD. We obtained p-values from BOLT-LMM using a two-sided test. The green dashed line is the genome-wide significant level (P < 5 × 10 − 8). Credit: Nature Genetics (2023). DOI: 10.1038/s41588-023-01372-4
A team of medical researchers, engineers and computer scientists affiliated with multiple institutions across the U.S. has found that machine learning technology can help doctors predict which patients are at risk of developing COPD. In their study, reported in the journal Nature Genetics, the group trained a deep-learning network using patient spirogram data to predict the development of COPD.
COPD is the third most common cause of death worldwide. The term describes a large number of obstructive lung disorders such as asthma, bronchitis and emphysema. Prior research has shown that the earlier COPD is treated, the sooner therapies can be applied, slowing its progression. For that reason, medical scientists have worked hard to find new ways to spot patients most at risk. In this new effort, the research group applied machine learning to the task.
The researchers trained a deep convolutional neural network to recognize the difference between people with COPD and those who do not have it. Data to teach the system came from patient medical records, potential diagnosis classification systems and spirograms. Spirograms are created by administering spirometry to patients, in which patients blow into a tube-like device that is connected to a machine that calculates lung strength.
Once the system could distinguish healthy lungs from those with COPD, the team added liability score data that has been compiled over many years to help spot early COPD in patients. They then ran the system on data from 325,000 patients in the UK Biobank, which included spirograms. And they also fed it risk data from participants in several other health care–related initiatives. They found that they were able to train the system to detect very early signs of COPD in patients.
The team concludes by suggesting that their system could soon be used to screen patients for COPD by feeding it spirogram data. They also note that it could be used in new research efforts aimed at more fully understanding how COPD gets started in the lungs and why it sometimes progresses so quickly.

Leave a Reply