by Bob Yirka, Medical Xpress
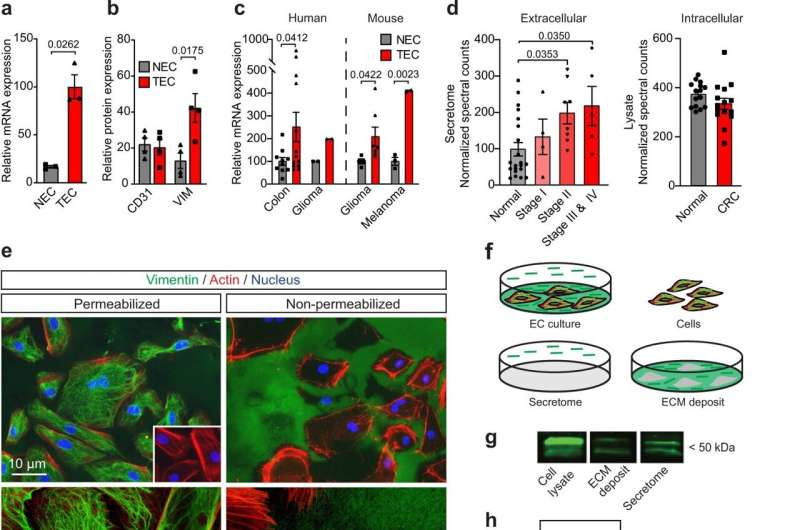
Vimentin is overexpressed in tumor endothelial cells and is present extracellularly. a, b Vimentin mRNA (a; n = 3; qPCR) and protein (b; n = 4; flow cytometry) expression in isolated endothelial cells (EC) from human colon tumor (TEC) and normal colon (NEC). c Vimentin mRNA expression in isolated EC from human (colon, n = 13; glioma, n = 2) and murine (glioma, n = 7; melanoma, n = 2) tumors. d Proteomics analysis of human normal colon and colorectal cancer tissues for extracellular vimentin in secretome (left panel; n = 21 (normal), n = 4 (Stage I), n = 8 (Stage II), n = 5 (Stage III & IV)) and total intracellular vimentin (right panel; n = 15 (normal), n = 15 (CRC Stage I–IV)). Data are presented as mean ± SEM in a–d. p values represent paired t test (a, c, d right panel), unpaired t test (b), and one-way ANOVA (d left panel). e Immunofluorescent staining of fixated and permeabilized HUVEC (left panels) and live intact HUVEC (right panels). Inset: negative control. Representative images of at least three independent experiments are shown. f Schematic representation of vimentin localization (in green). g Western blotting of total cell lysate, ECM deposit, and secretome of HUVEC. Representative sections of at least three independent experiments are shown. h Global proteomics analysis (n = 1) of HUVEC lysate, secretome, and ECM deposit. i (Left) Proportion of known tumor EC markers (TEC, red) among externalized proteins. (Right) Secretion mechanisms among externalized proteins. j Protein–protein interaction analysis using STRING of externalized TEC markers. Opacity levels of the nodes are proportional to secretion abundance. k Effect of angiogenesis inhibitors and cytokines on vimentin secretion. Relative secretion is color-coded according to the legend right of the panel, and agent types are color-coded according to the legend below the panel. l Schematic of different cellular protein secretion pathways. m Effect of different protein secretion mediators on vimentin secretion. Legend as in k. Data are color-coded as mean values of relative secretion in k and m; numbers of samples are presented in the Source Data file. *p < 0.05 based on Kruskal–Wallis test with Dunn’s multiple comparison test correction for k and m. Source data are provided as a Source Data file. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-30063-7
A team of researchers affiliated with multiple entities in the Netherlands and one in Switzerland reports that a vaccine targeting a certain protein is effective in treating multiple types of cancers in several kinds of animals. In their paper published in the journal Nature Communications, the group describes the protein they discovered that is unique to cancer tumors and the vaccine they developed to treat patients.
In 2006, the same research team found that a protein called vimentin is produced in cancerous tumors and exists only in the blood that feeds such tumors. Subsequent work showed that the protein assisted with the creation of new blood vessels that enable tumor growth and that it also played a role in turning off the immune response. The researchers then developed a vaccine that would prevent the creation of vimentin inside tumors. They then tested the vaccine, called Griffioen, on several different laboratory animals with skin, brain and colorectal cancer and found varying degrees of success. Most recently, they tested it with dogs that had developed bladder cancer independently.
The researchers treated 35 of the dogs with their vaccine. Half of them survived to the end of the 400-day test period, and two of them fully recovered. They also treated one of their older pet dogs who had developed bone cancer and was expected not to live very long. The tumors disappeared and the dog returned to normal health soon thereafter.
The researchers conclude that their testing provides further proof that vimentin blocks the immune system and promotes the development of new blood vessels in tumors. And blocking it, they note, appears to be an effective therapy for the treatment of tumors. They note that thus far, they have seen no side effects with administration of Griffioen and report that more testing is required before clinical trials in humans can begin.

Leave a Reply