By Dr. Sanchari Sinha Dutta, Ph.D.
Reviewed by Dr. Jennifer Logan, MD, MPH
Skip to:
What are Schwann cells?
How do Schwann cells form the myelin sheath?
Schwann cells and axon regeneration
Pathophysiology related to Schwann cell
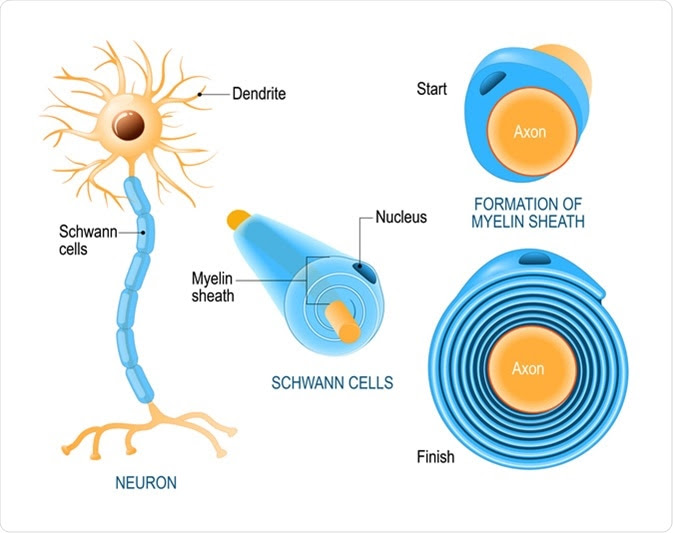
Schwann cells are a type of glial cells of the peripheral nervous system that help form the myelin sheath around the nerve fibers.
What are Schwann cells?
Schwann cells are derived from the neural crest and play crucial roles in the maintenance and regeneration of the motor and sensory neurons of the peripheral nervous system (PNS). They are mainly required for insulating (myelinating) and supplying nutrients to individual nerve fibers (axons) of the PNS neurons. The axonal conduction velocity increases due to myelination, which in turn is required for sending proper electrical signals through the nervous system. Schwann cells are absolute prerequisite for regenerating neurons of the PNS. There are also non-myelinating Schwann cells, which are required to provide nutrition and cushioning effects to non-myelinated axons.
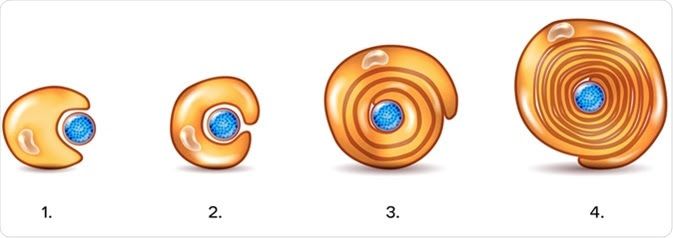
Regarding development of Schwann cells, the neural crest consisting of multipotent cells are differentiated into Schwann cell precursors, which then migrate and proliferate along the PNS axonal tracts. Afterward, the precursor cells undergo cellular differentiation to produce immature Schwann cells, which ultimately convert to either myelinating or non-myelinating Schwann cells.
Both myelinating and non-myelinating Schwann cells are covered by a basal lamina. The outside of the lamina is surrounded by a layer of connective tissue namely the endoneurium, which contains blood vessels, fibroblasts, and macrophages. Whereas, the inner surface of the lamina faces the Schwann cell plasma membrane.
How do Schwann cells form the myelin sheath?
The plasma membrane of Schwann cells, which contains high amount of lipid, forms the myelin sheath. The cholesterol present in the plasma membrane is essential for assembling the myelin sheath. Both survival and maturation of Schwann cell precursors as well as the extent of myelination depend on the expression of Neuregulin-type III on the axonal surface.
Schwann cells myelinate the axons with large diameter that transmit electrical signals at the highest speed. In contrast, slow-conducting axons are arranged together to form bundle-like structures, which are engulfed by non-myelinating Schwann cells.
Schwann cells and axon regeneration
Schwann cells play a vital role in axon regeneration. Any injury to the axon can lead to cell death and axonal degeneration. Upon injury, Schwann cells and macrophages are recruited to the injury site to remove dead cells and promote axonal regeneration. Mechanistically, Schwann cells promote the recruitment of macrophages by inducing myelin breakdown and increasing the expression of cytokines, such as tumor necrosis factor-alpha. In addition, Schwann cells induce axon regeneration and nerve cell survival by increasing the expression of a wide variety of growth factors, such as neurotrophins, transforming growth factor-beta, glial cell line-derived neurotrophic factor, epidermal growth factors, and platelet-derived growth factor. They also secrete extracellular matrix molecules, such as laminin and collagen, and cell adhesion molecules to support the regeneration process. The axonal growth is guided by the cues provided by the lumen of the basal lamina of Schwann cells.
Pathophysiology related to Schwann cell
Impaired Schwann cell functioning is mainly associated with demyelinating diseases, such as multiple sclerosis. In the PNS, various insults, including genetic mutations, trauma, autoimmune responses, and infections, can impair the myelination process and affect the functions of Schwann cells and axons. This can eventually lead to neurodegeneration.
Many peripheral demyelinating disorders, including Charcot-Marie-Tooth disease (CMT), are associated with structural and functional disruption of Schwann cells. In CMT, PMP22 is the most commonly affecting protein, which causes Schwann cell growth arrest and reduction in Schwann cell number. This condition is characterized by segmental demyelination and remyelination that is associated with reduced nerve conduction velocity.
Diabetic neuropathy is another disorder associated with Schwann cell damage in both sensory and motor nerves. Hyperglycemia (high blood glucose level) associated with diabetes mellitus is the main factor to induce damage to Schwann cells. The loss of trophic support from Schwann cells subsequently increases the risk of neurodegeneration.
Apart from demyelinating diseases, several neoplastic diseases are associated with Schwann cell damage. Such diseases include Schwannomas, neurofibromas, and malignant peripheral nerve sheath tumors. Of these diseases, Schwannomas are typically associated with neoplastic Schwann cell lesions; however, the condition is benign in nature and do not invade the adjacent nerve fibers.
Sources
NCBI. 2019. Histology, Schwann cells. https://www.ncbi.nlm.nih.gov/books/NBK544316/
Science Direct. 2015. Schwann cell. https://www.sciencedirect.com/topics/neuroscience/schwann-cell
Jessen KR. 2019. The Success and Failure of the Schwann Cell Response to Nerve Injury. Frontiers in cellular neuroscience. https://www.frontiersin.org/articles/10.3389/fncel.2019.00033/full
Bhatheja K. 2006. Schwann cells: Origins and role in axonal maintenance and regeneration. The International Journal of Biochemistry and Cell Biology. www.sciencedirect.com/science/article/abs/pii/S1357272506001634

Leave a Reply