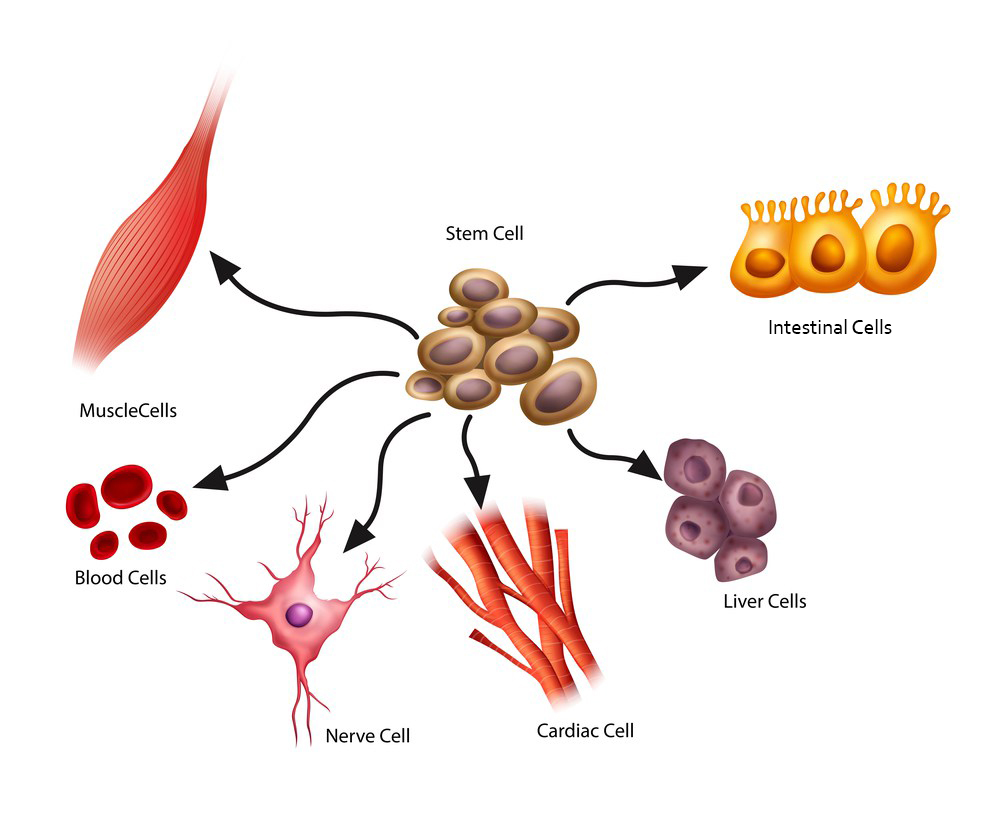
tem cells can multiply (self-renew) and differentiate into every cell within the human body, giving them enormous potential for use in regenerative medicine.
In a developing embryo, stem cells can differentiate into all of the specialized embryonic tissues. In adult humans, stem and progenitor cells act as a repair system for the body, replenishing specialized cells.
Stem cell research has been going on for over 50 years, because stem cells have a unique ability to divide and replicate repeatedly. In addition, their unspecialized nature allows them to become a wide variety of tissue types, which gives them enormous potential for use as living cell therapies.
What Types of Stem Cells Exist?
Several broad categories of stem cells exist, including:
- Embryonic Stem Cells (ESCs) – This is the only controversial stem cell type. ESCs are derived from blastocysts, a stage in the developing embryo. They can become any cell type within the human body.
- Perinatal Stem Cells – These cells are obtained during the period immediately before and after birth. Collection of these cell types does not impact the development of the fetus or newborn, so they are non-controversial.
- Adult Stem Cells – These are non-controversial cells found in living adults. Everyone has stem cells present in their bone marrow, fat (adipose tissue), and many other sites.
- Induced Pluripotent Stem Cells (iPS Cells) – iPS cells were discovered in 2006. They are non-controversial, because they are adult cells that are genetically reprogrammed in a lab. Like embryonic stem cells, they can become any cell within the body.
- Cancer Stem Cells (CSCs) – Cancer stem cells are a type of stem cell that biotech and pharma companies are exploring, because they play a role in facilitating the formation of tumors. Companies exploring CSCs are interested to discover how to manage and prevent cancer.
Originally, all stem cells were classified as either adult stem cells or embryonic stem cells (ESCs). However, a diverse range of stem cell types have since been identified. When iPS cells were discovered in 2006, the research community had a new stem cell type that possessed most of the characteristics of ESCs without the controversy.
Stem Cells Throughout the Human Lifecycle
To make things simple, apply these definitions to classify stem cells by when they are collected during the human lifecycle:
- Embryonic stem cells– Stem cells derived from embryos (controversial)
- Pre-natal stem cells– Stem cells derived from the fetus or supporting structures (non-controversial)
- Post-natal stem cells– Stem cells derived from a recent newborn (non-controversial)
- Adult stem cells– Stem cells derived from living humans (non-controversial). Common adult stem cell types include mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and neural stem cells (NSCs), among others.
Totipotent vs. Pluripotent vs. Multipotent
To understand the functional potential of each stem cell type, scientists like to describe to what degree each stem cell type can differentiate into other cell types.
When assessing the functional potential of stem cells, use the following definitions:
- Totipotent stem cells– Cells that have the capacity to form an entire organism
- Pluripotent stemcells – Can give rise to most, but not all, tissues within an organism
- Multipotent stem cells – Undifferentiated cells that are limited to giving rise to specific populations of cells
Human embryonic stem cells (hESCs) are totipotent cells that are derived from embryos that have been created in vitro at fertility clinics with informed donor consent. Embryonic stem cells are typically collected shortly after fertilization (within 4-5 days). At 5-6 days post-fertilization, embryonic stem cells begin to specialize, at which point they become pluripotent or multipotent cells.
Pluripotent and multipotent stem cells have a more limited differentiation capacity than totipotent stem cells. For example, multipotent blood stem cells can differentiate into red cells, white cells and platelets in the blood, but they cannot become any cell type.
The precise point at which a stem cell switches from a totipotent stem cell to a pluripotent or multipotent stem cell is often unclear. Furthermore, iPS cell technology allows us to reverse mature cell types back into a totipotent state. iPS cells are totipotent, so stem cells can now be collected at any point of the human lifecycle.
How Are Stem Cells Being Used in Medicine?
Today, most clinics that off stem cell treatments administer mesenchymal stem cells (MSCs), which they source from fat tissue or bone marrow. Mesenchymal stem cells are a type of multipotent stem cell that are being explored for use in orthopedic repair, pain management, arthritis, asthma, and many other applications. MSCs tend to exert effects on other cells and tissues within the human body, which is called “paracrine signaling.”
Although risks will exist whenever cell therapies are administered to humans, a large body of scientific evidence suggests that MSCs can be safe for patient use when properly administered and monitored. There is an additional layer of safety that occurs when cells are multipotent (limited in their differentiation capacity). Often, it is safer for them to be self-derived (“autologous”), rather than from someone else (“allogeneic”).
Another stem cell type that is commonly used is the hematopoietic stem cell (HSC). HSC transplantation has been used for decades as a means of rebuilding the immune system after a patient undergoes radiation or chemotherapy.
What are the Risks of Stem Cells?
There are many companies working to introduce legitimate stem cell therapies into clinical practice. Unfortunately, there are also unregulated stem cell groups that are offering unverified and unsafe stem cell therapies to patients.
One of the risks of totipotent stem cells (embryonic stem cells and iPS cells) is that they have the potential to produce uncontrolled proliferation. The biggest concern surrounding the clinical application of these cells is their tendency to form tumors. Pluripotent and multipotent stem cells have a lower risk of producing tumor formation, but can potentially create growth of the wrong tissue type for a given location within the human body. Additionally, iPS cells are artificially manipulated in a laboratory process, so there is the possibility that the cells can act in unexpected ways.
Because many of these risks can be mitigated and monitored, stem cells are currently being investigated in hundreds of clinical trials worldwide. The majority of these clinical trials involve the use of mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs). You can view approximately three-quarters of active stem cell trials worldwide by visiting ClinicalTrials.gov. Additional trials can be found on a country-by-country basis.
To make things more complex, the U.S. FDA regulates stem cell treatments with as two different types, commonly called “361” and “351” products:
- 361 products – 361 products are not required to be licensed or approved by the FDA and are regulated under Section 361 of the Public Health Service (PHS) Act.
- 351 products – 351 products do require clinical trials to demonstrate safety and efficacy and are regulated as a “drug, device, or biological product” under the Federal Food, Drug, and Cosmetic Act (FDCA) and Section 351 of the PHS Act.
