Claudia López Lloreda
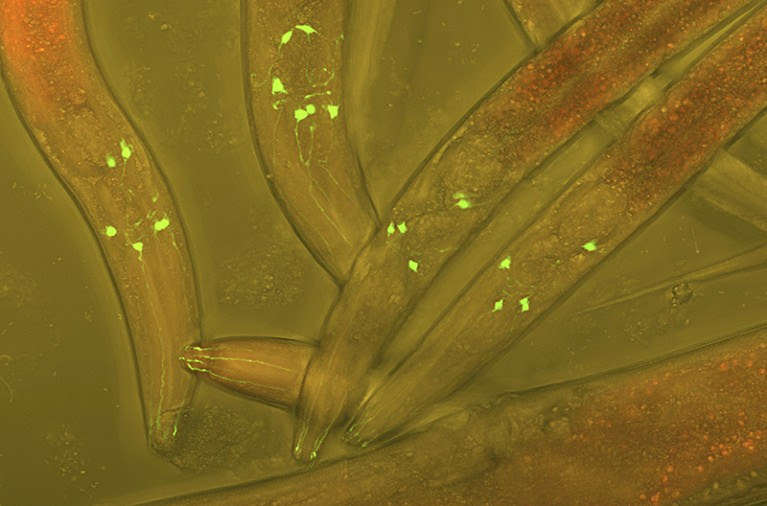
The worm Caenorhabditis elegans has 302 neurons (green) that researchers can study using tools such as fluorescent markers.
Credit: Heiti Paves/Science Photo Library
The idea that the nervous system passes messages from one nerve cell to another only through synapses — the points where the cells link up end to end — is changing. Two studies show how messages can pass between cells over longer distances, through a ‘wireless’ nerve network in the worm Caenorhabditis elegans.
Researchers had not appreciated the extent of this wireless communication, which happens when a molecule called a neuropeptide is released by one neuron and intercepted by another some distance away. The new studies, published in Nature1 and in Neuron2, map out the entire network of neuropeptide communication in a model organism for the first time. “We knew that these chemical connections existed, but this is probably the most comprehensive study in an entire nervous system,” says Gáspár Jékely, a neuroscientist at Heidelberg University in Germany who was not involved in the work. And what the research shows, he adds, is that “it’s not all about the synapses”.
Map-makers
Researchers had previously worked out anatomical wiring maps — connectomes — showing how all the neurons in the fruit fly (Drosophila melanogaster) and in C. elegans are linked by their synapses. However, William Schafer, a neuroscientist at the MRC Laboratory of Molecular Biology in Cambridge, UK, wondered about the role of neuropeptides, which had been considered merely helpers in nervous-system messaging. “When I first started talking about this,” he says, “some people wondered, ‘is it all just kind of a soup’” where neuropeptides randomly float from one neuron to the next, “or can you really think about it like a network?”
He and his colleagues analysed which neurons in the C. elegans nervous system expressed genes for certain neuropeptides and which ones expressed genes for the receptors of those neuropeptides. Using this data, the team predicted which pairs of nerve cells might be communicating wirelessly. On the basis of these results, the researchers generated a potential map of wireless connections in the worm, finding dense connectivity that looks very different from the anatomical wiring diagram of C. elegans. They published their findings in Neuron2 last week.
Independently, a team led by Andrew Leifer, a neuroscientist at Princeton University in New Jersey, went about studying how signals travel through C. elegans by measuring neuronal activity, which revealed the contribution of this wireless network. The team enlisted optogenetics, a technique that uses light and light-sensitive proteins to trigger nerve cells so that they send electrical ‘messages’’. One by one, the researchers activated each of C. elegans’ 302 neurons and then imaged how signals propagated from one neuron to the next.
Measurements of neural activity in the worm’s head as individual neurons are optically stimulated one-at-a-time, seen as dark red in this visualization.
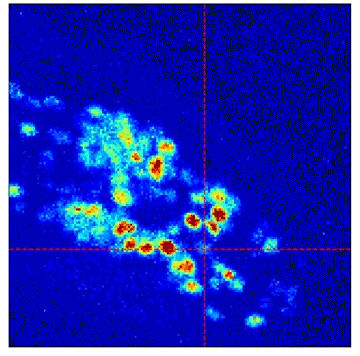
Researchers used optogenetics to stimulate each of C. elegans’ neurons (shown in cross-hairs here) and then watched how the electrical signal propagates to other nerve cells (red flickering). Credit: Francesco Randi, Princeton University
The map of activity they created did not follow what they would have predicted for C. elegans on the basis of its standard connectome alone — and they suspected that neuropeptide communication was the missing piece. So they produced a genetically engineered worm that lacked a protein crucial for this type of signalling, and saw that when they tried to activate the worm’s cells with optogenetics, many of them stayed silent. This suggests that wireless communication in the worm directly activates neurons.
When the researchers developed a model to describe neuronal activity in C. elegans, they found that one incorporating both wired, synaptic connections and wireless signalling better predicted how signals travelled in the worm than did the synaptic connections alone. The team published its results in Nature1 earlier this month and presented them at the Society for Neuroscience meeting in Washington DC on 14 November.
A whole new view
“It was surprising to see how much [neuropeptide] communication can actually lead to direct activation of neurons,” says Francesco Randi, first author of the Nature paper, who carried out the work while at Princeton.
“The neuropeptide network was thought of as a helper for synaptic signalling,” says Isabel Beets, a neuroscientist at the Catholic University of Leuven in Belgium and an author of the Neuron study. “But the extensive scale of this signalling map really shows that it’s equally important, complex and maybe even more diverse than the synaptic signalling network”.
Drugs such as the popular weight-loss treatment semaglutide (Wegovy) can activate neuropeptide receptors in the body, so understanding this wireless network is important, Schafer says. The next steps for Schafer and his colleagues will be to undertake similar studies in other organisms — aiming to understand how the neuropeptide network, in combination with the ‘wired’ synaptic network, contributes to an organism’s behaviour. A technique published in Science3 last week that allows researchers to visualize where neuropeptides bind to their receptors could aid in this quest. Because neuropeptides are conserved across species, some researchers suspect that this network could look similar to those in other organisms, including humans.
“The two papers are beautiful examples of taking advantage of one simple, well-studied organism with lots of molecular and genetic tools to start learning lessons that I am 100% positive are going to apply to all animals,” says Stephen Smith, a neuroscientist at the Allen Institute in Seattle, Washington.
Researchers hope the findings will spur others to think differently about how neural dynamics arise. “I think we have to move away from the synapse-only view of the nervous system,” Jékely says. “That’s just not going to work.”
doi: https://doi.org/10.1038/d41586-023-03619-w
References
Randi, F., Sharma, A. K., Dvali, S. & Leifer, A. M. Nature 623, 406–414 (2023).
Ripoll-Sánchez, L. et al. Neuron 111, 3570–3589 (2023).
Wang, H. et al. Science 382, eabq8173 (2023)

Leave a Reply