by Vanderbilt University Medical Center

Tet2-deficient macrophages are hyperinflammatory in early ischemic kidney injury. Credit: Nature Medicine (2024). DOI: 10.1038/s41591-024-02854-6
A U.S.-Canadian research collaboration led by Vanderbilt University Medical Center has identified common, age-associated changes in the blood as a risk factor for acute kidney injury (AKI), which occurs in more than 1 in 5 hospitalized adults worldwide.
This discovery, reported in the journal Nature Medicine, could open the door to new, more effective treatments for AKI and a way to prevent its progression to end-stage renal disease requiring kidney dialysis.
The focus of this investigation was clonal hematopoiesis of indeterminate potential (CHIP), somatic (noninherited) mutations in blood stem cells that can trigger explosive, clonal expansions of abnormal cells.
CHIP, which affects 10–20% of people ages 65 and older, is associated with an estimated 40% greater risk of death from cardiovascular, lung and liver disease, and other inflammatory conditions. This age group is also especially vulnerable to AKI.
“In addition to known causes for AKI, identification of an association with CHIP provides new insight into the increased risk and underlying mechanisms for the development of AKI among the older population,” said Raymond Harris, MD, co-corresponding author of the paper with Alexander Bick, MD, Ph.D.
“We commonly think about how a chronic inflammation caused by CHIP can cause chronic diseases, but I was quite surprised at the effect CHIP had on an acute inflammation,” Bick added.
Bick, an assistant professor of Medicine in the Division of Genetic Medicine, has pioneered methods for determining the presence of CHIP and the mechanisms by which it leads to disease.
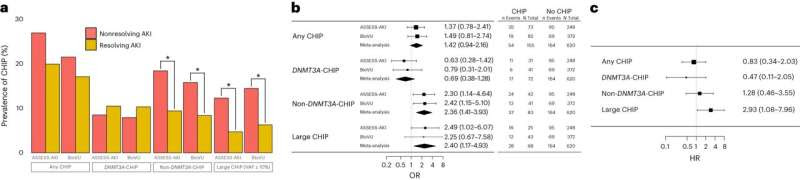
Blood mutations increase risk for acute kidney injury, says studyCHIP is associated with impaired recovery from AKI in the ASSESS-AKI and BioVU cohorts. a, Prevalence of CHIP among individuals with a resolving AKI pattern in ASSESS-AKI (n = 191) and BioVU (n = 88) compared to a nonresolving AKI pattern (n = 130 for ASSESS-AKI and n = 366 for BioVU), as defined by Bhatraju et al. b, Odds of nonresolving AKI pattern according to CHIP status, adjusted for age, sex, baseline creatinine, AKI stage, smoking status, ethnicity and history of diabetes, hypertension and cardiovascular disease. c, Risk of significant kidney function impairment (primary study composite outcome of ESKD or eGFR decline by ≥50%) over 5 years of follow-up among ASSESS-AKI participants with baseline AKI according to CHIP status, adjusted for age, sex, baseline creatinine, AKI stage, smoking status, ethnicity and history of diabetes, hypertension and cardiovascular disease. *P < 0.05, ***P < 0.001. Credit: Nature Medicine (2024). DOI: 10.1038/s41591-024-02854-6
Harris, the Ann and Roscoe R. Robinson Professor of Medicine, and director of research in the Division of Nephrology and Hypertension, is internationally known for his investigations of acute and chronic renal injury.
Researchers from Canada and across the United States contributed to the study, which was supervised by Bick, Harris and Cassianne Robinson-Cohen, Ph.D., associate professor of Medicine at VUMC. The study was led by first author Caitlyn Vlasschaert, MD, a resident physician in Medicine at Queens University in Kingston, Ontario, Canada, who was previously a visiting research scholar at VUMC.
The researchers first showed that CHIP was associated with AKI in a meta-analysis of three population-based cohorts totaling more than 440,000 individuals from the UK (United Kingdom) Biobank, and two long-running studies in the United States.
The CHIP-AKI association was more pronounced in patients who required dialysis and in individuals with mutations other than in the DNMT3A gene, including the Tet2 and Jak2 genes.
Pursuing this finding, the researchers created mouse models of CHIP in which both Tet2 and Jak2 were mutated. They observed more severe AKI, greater infiltration of pro-inflammatory macrophages, a type of immune cell, in the kidneys, impaired recovery from AKI, and greater post-AKI fibrosis, or scarring.
These results suggest that CHIP inhibits recovery from AKI by inducing a pro-inflammatory response by mutant, infiltrating macrophages. It therefore may be possible to reduce this risk by targeting elements of the innate immune system that initiate and fan the flames of inflammation in the kidney, the researchers concluded.
“It was a privilege to closely collaborate with Dr. Harris’s research group on this effort,” Bick said. “It was remarkable to see how well our biobank scale observations were recapitulated by Dr. Harris’s mechanistic experiments.”
More information: Caitlyn Vlasschaert et al, Clonal hematopoiesis of indeterminate potential is associated with acute kidney injury, Nature Medicine (2024). DOI: 10.1038/s41591-024-02854-6
Provided by Vanderbilt University Medical Center

Leave a Reply