by Max Delbrück Center for Molecular Medicine
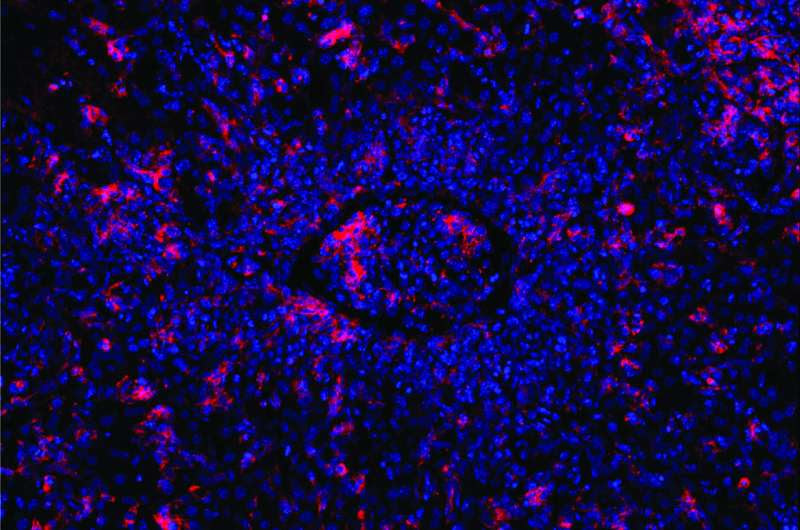
Mutated T cells are unable to kill B cells (red) induced by the Epstein-Barr virus. This causes other immune cells to flow into the area of infection, thereby blocking a blood vessel (center). Credit: Elijah D. Lowenstein and Xun Li, K. Rajewsky Lab, Max Delbrück Center
Some hereditary genetic defects cause an exaggerated immune response that can be fatal. Using the CRISPR-Cas9 gene-editing tool, such defects can be corrected, thus normalizing the immune response, as researchers led by Klaus Rajewsky from the Max Delbrück Center now report in Science Immunology.
Familial hemophagocytic lymphohistiocytosis (FHL) is a rare disease of the immune system that usually occurs in infants and young children under the age of 18 months. The condition is severe and has a high mortality rate. It is caused by various gene mutations that prevent cytotoxic T cells from functioning normally. These are a group of immune cells that kill virus-infected cells or otherwise altered cells.
If a child with FHL contracts a virus—such as the Epstein-Barr virus (EBV), but also other viruses—the cytotoxic T cells cannot eliminate the infected cells. Instead, the immune response gets out of control. This leads to a cytokine storm and an excessive inflammatory reaction that affects the entire organism.
“Doctors treat FHL with a combination of chemotherapy, immunosuppression and bone marrow transplantation, but many children still die of the disease,” says Professor Klaus Rajewsky, who heads the Immune Regulation and Cancer Lab at the Max Delbrück Center.
He and his team have therefore developed a new therapeutic strategy. Using the CRISPR-Cas9 gene-editing tool, the researchers succeeded in repairing defective T cells from mice and from two critically ill infants. The repaired cytotoxic T cells then functioned normally, with the mice recovering from hemophagocytic lymphohistiocytosis.
Gene repair strategy works in mice
The starting point for the study were mice in which the team could mimic EBV infections. In these animals, the researchers altered a gene called perforin so that its function was completely lost or severely compromised—a common genetic defect in patients with FHL.
When they then elicited a condition resembling an EBV infection, the affected B cells multiplied uncontrollably because the defective cytotoxic T cells were unable to eliminate them. As a result, the immune response went into overdrive and the mice developed hemophagocytic lymphohistiocytosis.
The team next collected T memory stem cells—that is, long-lived T cells from which active cytotoxic T cells can mature—from the blood of the mice. The researchers used the CRISPR-Cas9 gene-editing tool to repair the defective perforin gene in the memory T cells and then injected the corrected cells back into the mice. The immune response in the animals quietened down and their symptoms disappeared.
How long protection lasts is uncertain
The first author of the paper, Dr. Xun Li, used blood samples from two sick infants to test whether the strategy also works in humans. One had a defective perforin gene, the other a different defective gene.
“Our gene repair technique is more precise than previous methods, and the T cells are virtually unchanged after undergoing gene editing,” says Li. “It was also fascinating to see how effectively the memory T cells could be multiplied and repaired from even a small amount of blood.”
More information: Xun Li et al, Precise CRISPR-Cas9 gene repair in autologous memory T cells to treat familial hemophagocytic lymphohistiocytosis, Science Immunology (2024). DOI: 10.1126/sciimmunol.adi0042. www.science.org/doi/10.1126/sciimmunol.adi0042
Journal information: Science Immunology
Provided by Max Delbrück Center for Molecular Medicine

Leave a Reply