by Marina Romero-Ramos, Aarhus University
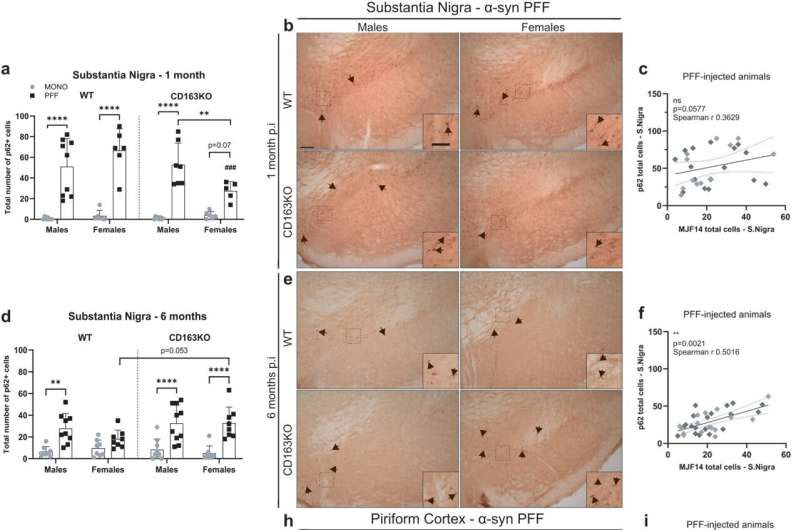
Differential expression of p62/SQSTM1 autophagy marker in the substantia nigra, piriform cortex and amygdala after α-syn PFF injection. Bar graphs with individual points illustrate the total number of p62+ cells in the SN at a 1 and d 6 months p.i. b Representative images of p62/SQSTM1 immunostaining in α-syn PFF animals at b 1- and e 6 months p.i. in the SN, h piriform cortex and k amygdala. g Bar graphs with individual points illustrate the percentage of area covered by p62+ staining 6 months after α-syn PFF-injection in WT/CD163KO males and females in the piriform cortex and j amygdala. The number of p62+ cells correlated to MJF14+ cells in the SN at c 1- and f 6 months p.i. The percentage of area covered by p62+ staining correlated to the one covered by pSer129+ staining i in the piriform cortex and l amygdala at 6 months p.i. in all α-syn PFF animals. In the correlation plots: light gray symbols represent females and dark gray males. m Representative images of p62/SQSTM1 immunostaining of amygdala and piriform cortex from PBS or α-syn MONO injected animals. Scale bar represents 100 and 50 μm in cropped images in (b, e) and 50 μm in (h, k). Values are mean ± SEM (n = 6–10). Statistics: Spearman two-tail p values (*<0.05, **<0.01, ***<0.001, ****<0.0001), Spearman r and best-fit slope with 95% confidence intervals are plotted. Two-way ANOVA followed by Sidak’s multiple comparison test. *p < 0.05, **<0.01, ***<0.001, ****<0.0001. Credit: npj Parkinson’s Disease (2023). DOI: 10.1038/s41531-023-00606-w
More men than women are diagnosed with Parkinson’s disease. The reason why is still followed by a big question mark, but the sex difference is nonetheless a growing area of interest for researchers.
Now, a group of researchers from Aarhus University, led by Professor Marina Romero-Ramos, might have found one of the pieces to this puzzle.
In an article recently published in npj Parkinson’s Disease, the researchers shed light on a specific receptor called CD163, a protein expressed mainly in blood-immune phagocytic cells. The protein is involved in the immune response during the neurodegenerative process associated with the aggregation of a-synuclein in Parkinson’s disease, and it seems to play a specific and protective role in the body’s defense against the damages related to the disease.
“Our study suggests that CD163 is involved in the mechanism controlling the entrance of lymphocytes in the brain during neurodegeneration,” Romero-Ramos explains.
Most interestingly, the protein seems to exert a neuroprotective role particularly relevant in females.
The findings thus add new insight into the sex difference related to Parkinson’s disease and suggest that some of the answers might be expressed in the body’s protection system.
“We believe that the sex differences observed in the risk to develop PD, higher in males, as well as the disparities in the disease presentation between sexes might be due to differences in the immune response,” Romero-Ramos explains.
This study provides evidence that the increased expression of CD163 in patients with Parkinson’s disease might be a compensatory mechanism aiming at the protection of neurons—especially in females. Romero-Ramos hopes that the study will increase research focus on both the immune system and the involvement of sex differences in the disease
More information: Sara A. Ferreira et al, Sex-dimorphic neuroprotective effect of CD163 in an α-synuclein mouse model of Parkinson’s disease, npj Parkinson’s Disease (2023). DOI: 10.1038/s41531-023-00606-w
Journal information: npj Parkinson’s Disease
Provided by Aarhus University

Leave a Reply