by University of Montreal
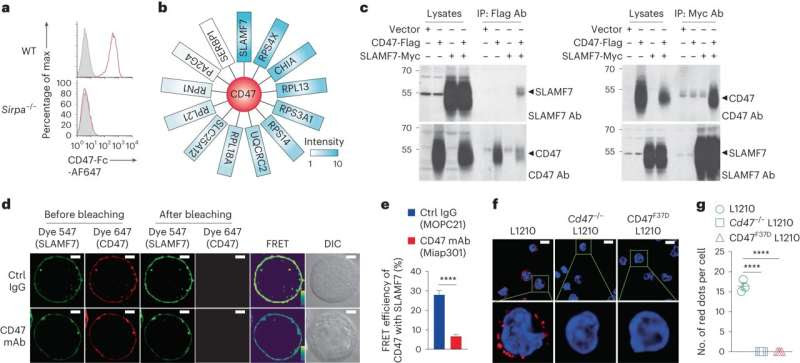
CD47 interacts in cis with SLAMF7 on tumor cells. a, Binding of CD47-Fc (red line) to WT or Sirpa−/− BMDMs assayed by flow cytometry. Filled curves, Ctrl Fc. b, Mass spectrometry analysis of Flag mAb immunoprecipitates from L1210 derivatives expressing a Flag-tagged variant of mCD47 showing various potentially associated proteins. c, Co-IP assay of CD47 and SLAMF7 in CD47−/− 293T cells expressing a Flag-tagged variant of mCD47 and a Myc-tagged variant of mSLAMF7. IP, immunoprecipitation. d,e, Representative confocal microscopy images (d) and compiled data from 18 cells in three independent experiments (e) of FRET assays of mCD47 (acceptor) and mSLAMF7 (donor) in CD47−/− 293T cells in the presence of Fc-silent Ctrl IgG (MOPC21) or CD47 mAb (Miap301) assayed by confocal microscopy. Pseudo-color, the yellow to purple spectrum denotes strong to weak FRET. Scale bars, 5 μm. f,g, Representative confocal microscopy images. Credit: Nature Immunology (2023). DOI: 10.1038/s41590-023-01671-2
Major work led by Dr. André Veillette’s team at the Institut de recherches cliniques de Montréal (IRCM), in collaboration with a group of researchers, and just published in Nature Immunology, managed to identify a previously unknown molecular action that prevents phagocytosis, which is a process that promotes the immune system’s response to cancer. A Research Briefing on the work done by the team has been published in the same journal.
Macrophages are cells of the immune system. One of the roles of macrophages is to engulf, or “eat,” cells that are defective or dangerous, including cancer cells. This process is named phagocytosis. Macrophages can be called into action to eliminate cancer cells. However, this capacity is often defective, because macrophages are put in a state of dormancy by the cancer cells.
This is in part because a particular molecule called CD47 is often over-abundant on cancer cells. CD47 prevents phagocytosis by triggering a molecule or “receptor” on macrophages named SIRPα. Agents that block the ability of CD47 to trigger SIRPα have shown promising results for treating cancer.
The research team collaborated in the identification of a previously unknown way by which CD47 prevents phagocytosis. They found that CD47 also blocks the molecule SLAMF7 on blood cancer cells, such as multiple myeloma and lymphoma. The researchers developed a new antibody, named Z10, that frees SLAMF7 from CD47, thereby augmenting elimination of tumor cells. When combined with other agents Z10 is highly effective against cancer cells expressing SLAMF7, at least in mice.
Why this research matters
Cancer remains the leading cause of death in Canada. It is estimated that 2 out of 5 Canadians will be diagnosed with cancer in their lifetime, and approximately 1 in 4 will die from the disease. The battle against cancer is thus still raging on several fronts, and the deep understanding of the role of the immune system in the growth of cancer cells is a major component of this battle.
These data suggest that Z10 is an effective new treatment for human cancers expressing SLAMF7, including multiple myeloma and lymphoma. The Veillette team is now setting up partnerships with pharmaceutical companies to test Z10 in clinical trials.
This study was done in collaboration with the laboratory of Enfu Hui, an expert in protein–protein interactions and immune regulation.
More information: Zhenghai Tang et al, CD47 masks pro-phagocytic ligands in cis on tumor cells to suppress antitumor immunity, Nature Immunology (2023). DOI: 10.1038/s41590-023-01671-2
Combining a first-in-class SLAMF7 antibody with SIRPα blockade for anti-tumor immunity, Nature Immunology (2023). DOI: 10.1038/s41590-023-01673-0
Journal information: Nature Immunology

Leave a Reply