NEWS FEATURE
15 May 2024
By Carissa Wong
Researchers are using new molecules, engineered immune cells and gene therapy to kill senescent cells and treat age-related diseases.
Illustration: Paweł Jońca
Lurking throughout your body, from your liver to your brain, are zombie-like entities known as senescent cells. They no longer divide or function as they once did, yet they resist death and spew out a noxious brew of biological signals that can slow cognition, increase frailty and weaken the immune system. Worst of all, their numbers increase as you age.
For more than a decade, researchers have been trying to see whether they can selectively destroy these cells with a variety of drugs. In a pivotal study1 published in 2015, a team at the Mayo Clinic in Rochester, Minnesota, and at the Scripps Research Institute in Jupiter, Florida, discovered that a combination of two compounds, called dasatinib and quercetin, killed senescent cells in aged mice. The treatment made the mice less frail, rejuvenated their hearts and boosted their running endurance. The finding opened the door to a new area of medicine called senolytics.
Now, fresh results from animal studies and human clinical trials have added momentum to the field. In mice and monkeys, researchers are using genetic tools to reprogram and kill senescent cells. Others are engineering senolytic immune cells. And about 20 clinical trials are ongoing. Researchers are testing new and repurposed drugs that could have senolytic properties, in the hope of combating age-related conditions, including Alzheimer’s disease, lung disease and chronic kidney disease.
“I am convinced that senolytics will have an impact in the clinic,” says Anirvan Ghosh, chief executive of Unity Biotechnology, a company in South San Francisco, California, that is developing senolytics. “I think the question is really what the agent looks like and what the first approved drug is.”
Zombie cells
Senescent cells were first described in 1961 by US biologists Leonard Hayflick and Paul Moorhead, who discovered that human cells in a laboratory dish will divide no more than about 50 times before either dying or entering the twilight state of cell senescence2. In the lab, it can take weeks for dividing cells to become senescent. But researchers have yet to uncover how much time this process takes in the body, how long senescent cells last and whether all cell types can become senescent.

Hacking the immune system could slow ageing — here’s how
Beyond hitting the limits of cell division, cell senescence can arise owing to other factors such as physical injury, malnourishment or DNA damage caused by UV light. Researchers initially thought that it evolved to prevent damaged cells from replicating uncontrollably and causing cancer. This might be the case to some extent, but it didn’t make sense that the cells would stick around in the body instead of simply dying, such as through the controlled programme of cell death known as apoptosis.
Researchers eventually discovered that senescent cells were avoiding apoptosis so they could perform a service, belching out a potent mix of inflammatory signals — including the cytokines interleukin-6 and interferon-γ — that prompt the immune system to clear out damaged cells. This helps to make room for damaged tissues to regenerate and repair.
The process works well until the immune system weakens with age, leading to a build-up of senescent cells that stir up excessive inflammation. Researchers have found that an accumulation of senescent cells and age-related inflammation correlates with many diseases, including osteoporosis, diabetes, heart disease, kidney disease and Alzheimer’s disease. For many scientists in the field, this realization prompted a shift away from understanding what the cells are doing to working out how to kill them.
Tipping the balance
One key strategy in senolytics involves designing drugs that stop senescent cells from resisting apoptosis. Usually, the cells survive by producing anti-death proteins. Blocking these with drugs can force the cells to succumb to death.
Unity Biotechnology is at the forefront of this approach, say researchers. In a February study3, Ghosh and his colleagues found that senescent cells were more abundant in the retinas of diabetic mice than in those of healthy mice. It was possible, the team predicted, that senescent cells in the blood vessels of the eye play a part in diabetes-related vision loss.

Why is exercise good for you? Scientists are finding answers in our cells
This condition, known as diabetic macular oedema, is caused by high blood sugar and makes those delicate blood vessels leaky, particularly in older individuals. The eye condition is a leading cause of blindness worldwide, estimated to affect 27 million adults. But around half of patients get little benefit from the standard treatment, which uses a cancer drug originally designed to slow down the growth of blood vessels. “There is an unmet need,” Ghosh says.
The researchers designed a drug, called foselutoclax, which blocks the action of BCL-xL, a key anti-death protein that is abundant in senescent cells. When they injected the drug into the eyes of diabetic mice, it killed senescent cells in the blood vessels supplying the retina, but not healthy cells3. “We see a very selective elimination,” says Ghosh.
The senolytic drug reduced the leakiness of retinal blood vessels in diabetic mice by around 50%. Moreover, the treated mice performed better in vision tests compared with controls. Next, the team turned to humans. In a phase II trial, researchers administered a single injection of foselutoclax into the eyes of about 30 people. Eleven months later, those treated with the senolytic could read 5.6 more letters, on average, on an optician’s chart compared with participants who had received a placebo treatment.
After just a couple of weeks, says Ghosh, one participant called him to say the treatment was making her life much easier. Another saw rapid improvements in their colour vision. The team expects to publish the results later this year, but in the meantime, Unity is running another phase II trial that will compare the senolytic with standard therapy.
Unity’s results are promising, say researchers. “I think within the next five years we may see this treatment for diabetic macular oedema being offered in the clinic,” says Sundeep Khosla, who studies ageing at the Mayo Clinic.
Rather than making senolytics from scratch, some scientists are testing drugs that already exist. These include dasatinib, which is approved in the United States as a cancer therapy, and two commercially available, plant-derived chemicals called quercetin and fisetin. The latter two are sold as supplements to dampen inflammation, boost brain health and reduce the risk of age-related disease. These claims are based on rodent studies in which the drugs have been shown to clear senescent cells and reduce inflammation
Do cutting-edge CAR-T-cell therapies cause cancer? What the data say
In a 2019 study5, researchers used dasatinib and quercetin to remove senescent brain cells in a mouse model of Alzheimer’s disease. Mice treated with the senolytics had reduced brain inflammation and improved memory compared with animals that were given a placebo. Spurred on by these promising data from mice, Miranda Orr at Wake Forest University School of Medicine in Winston-Salem, North Carolina, and her colleagues last year conducted the first safety trial of the drug combination in people with early stage Alzheimer’s disease.
Orr’s team gave five people dasatinib and quercetin intermittently for three months. The researchers found that the drugs were safe and that dasatinib was present in samples of cerebrospinal fluid, suggesting it could cross into the brain. Quercetin was not detected in brain fluid samples, but Orr says she suspects that it did reach the brain and was rapidly broken down. The team is now conducting a larger trial to track the cognition of people with and without Alzheimer’s disease for nine months after they take a placebo or the drug combination. The results should be released in 2025, says Orr.
Khosla says that fresh data should also emerge this year from the largest human trial of dasatinib and quercetin so far. In this study, which is currently under peer review, his team looked at the effect of senolytics on the bones of healthy women.
Immune killers
When it comes to killing cells in the body, the immune system could be of help. And some researchers have latched on to the idea of using genetically engineered immune cells called chimeric antigen receptor (CAR) T cells. These can target and kill specific cells on the basis of the molecules they display on their surface. CAR-T-cell therapies are currently approved as a treatment for various blood cancers.
Earlier this year, cell biologist Corina Amor at Cold Spring Harbor Laboratory in New York and her colleagues identified a protein marker, called uPAR, on senescent cells in the livers, fat tissues and pancreases of older mice6. The researchers created CAR T cells that were designed to kill senescent cells bearing the uPAR marker. After the team infused the engineered cells into the blood of old mice, there was a decline in the proportion of liver, pancreas and fat cells that were senescent.
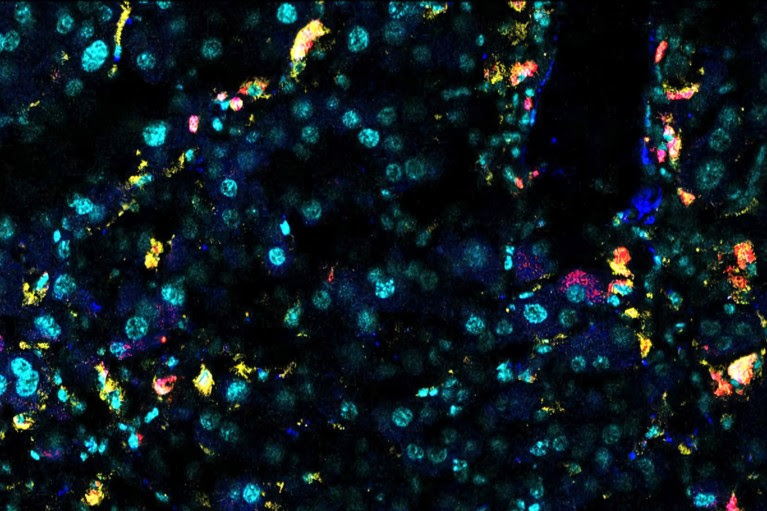
Senescent mouse liver cells express β-galactosidase (white) and uPAR (yellow).Credit: Memorial Sloan Kettering Cancer Center
Amor and her team found that old mice treated with the uPAR CAR T cells had reduced blood-sugar levels — a sign of improved metabolic health — and that the animals ran faster and for longer than did mice treated with non-engineered T cells, or with T cells that target a protein not found in mice. None of the mice treated with the senolytic CAR T cells showed signs that the T cells were toxic.
In young mice, the senolytic CAR T cells prevented age-related declines in blood-sugar regulation and exercise capacity. And in a March preprint7, the team reported that senolytic CAR T cells could rejuvenate the guts of old mice.
Still, further studies are needed to assess the safety of the therapy, says Amor. Moreover, it would be good to have an off switch for these cell-based drugs in case anything goes awry, she says. In rare cases, CAR T cells used to treat cancer in people seem to have become cancerous themselves.
Amor’s team plans to explore such safety switches in the near future. This would involve engineering the senolytic CAR T cells to carry a gene that induces cell death, which could be activated with a drug, she says. But CAR-T-cell therapies are expensive to make, says Robin Mansukhani, chief executive of Deciduous Therapeutics in San Francisco, which is also developing immune therapies against ageing.
Mansukhani is banking on a more affordable approach that harnesses a different kind of immune cell called a natural killer T cell. In 2021, researchers at Deciduous Therapeutics demonstrated8 the senolytic role of these cells, which naturally become less effective with age. They also found that drugs that can activate the immune cells helped to eliminate senescent cells in the damaged lungs of mice, reducing lung scarring and improving survival.
The researchers have developed a range of drugs that can bind to and supercharge natural killer T cells to treat various conditions, including diabetes and lung disease, says Mansukhani. Safety tests will be conducted in dogs and non-human primates later this year, and clinical trials should begin in the next two years, Mansukhani adds. The approach relies on smaller molecules that are easier to make than CAR-T-cell therapies, he says.
Gene therapy
Other teams are using gene therapy to kill senescent cells. In this approach, researchers package a gene that encodes a lethal protein called caspase-9 into fatty capsules studded with proteins derived from a virus. In mice and monkeys, the capsules have been found to deliver the gene to cells in the lungs, heart, liver, spleen and kidneys.

To stay young, kill zombie cells
Healthy cells are spared, because the gene is activated only in senescent cells that have high levels of one of two proteins called p16 and p53, says Matthew Scholz, chief executive at Oisín Biotechnologies in Seattle, Washington, which is developing the gene therapy. As a further safety switch, the lethal protein kicks off cell death only after the animal is given a very low dose of a drug called rapamycin, says Scholz. The researchers found that, over four months, a monthly dose of the therapy reduced frailty and cancer rates in old mice without causing harmful side effects. The comparison group involved mice that were given a placebo and low-dose rapamycin, says Scholz.
But a key limitation of this approach is that it relies on just one or two protein markers. Although p16 is widely used as a marker of senescence, definitive identification of cells in this state requires a panel of several markers. That means that, by targeting only p16 and p53, the gene therapy is probably eliminating some healthy, non-senescent cells that have these markers, and failing to kill some senescent cells that lack them, say researchers.
Better markers
Indeed, the issue of specificity is shared by all senolytic approaches, simply because there is more than one type of senescent cell. Researchers are only just beginning to uncover how many there are — and what markers they bear. “Without having really great biomarkers of senescent cells, it’s a little bit tricky to engage the right targets,” says Orr.
Orr is part of a large collaborative effort called the Cellular Senescence Network(SenNet), involving more than 200 researchers, that aims to produce atlases of senescent cells across the lifespan of humans and mice. Her team is using machine learning to improve definitions of brain-cell markers of senescence, then using them to map how senescent cells change with age and during dementia.
Ultimately, better markers of senescent cells will bring better senolytics that could one day prevent or treat age-related disease, she says. Ghosh echoes this optimism when it comes to killing zombie cells. “I think the fundamental science is so compelling that targeting senescent cells is definitely going to be of benefit.”
Nature 629, 518-520 (2024)
doi: https://doi.org/10.1038/d41586-024-01370-4
References
Zhu, Y. et al. Aging Cell 14, 644–658 (2015). Article : PubMed, Google Scholar
Hayflick, L. & Moorhead, P. S. Exp. Cell Res. 25, 585–621 (1961). Article : PubMed, Google Scholar
Crespo-Garcia, S. et al. Nature Med. 30, 443–454 (2024). Article : PubMed, Google Scholar
Yousefzadeh, M. J. et al. EBioMedicine 36, 18–28 (2018).Article : PubMed, Google Scholar
Zhang, P. et al. Nature Neurosci. 22, 719–728 (2019). Article : PubMed, Google Scholar
Amor, C. et al. Nature Aging 4, 336–349 (2024). Article : PubMed, Google Scholar
Eskiocak, O. et al. Preprint at bioRxiv https://doi.org/10.1101/2024.03.19.585779(2024). Article : PubMed, Google Scholar
Arora, S. et al. Med 2, 938–950 (2021). Article : PubMed, Google Scholar

Leave a Reply