by Nik Papageorgiou, Ecole Polytechnique Federale de Lausanne
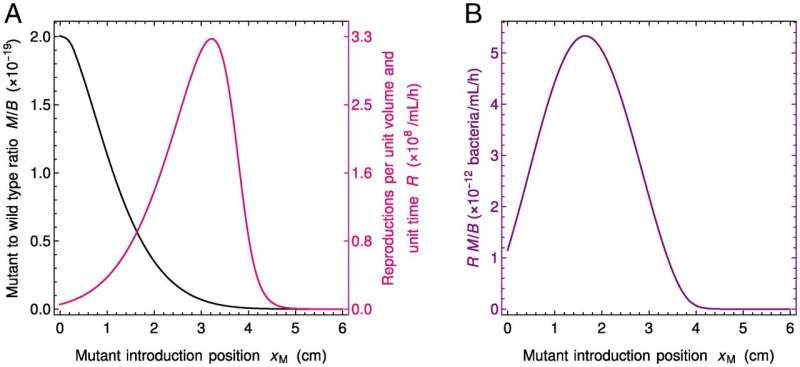
Fate of neutral mutants appearing at various locations in the gut. (A) Steady-state ratio M / B of mutant to wild-type bacteria concentrations and number of reproduction events R per unit volume and unit time vs. position xM of the mutant introduction. The ratio M / B yields the fixation probability of a mutant that appears at a given position xM in the system. As mutants generally appear upon division, the appearance of new mutants is proportional to R, which thus also matters for the overall likelihood that a mutant appears and fixes. (B) Product of the ratio M / B and the number R of reproductions per unit volume and unit time vs. xM. This quantity yields the fixation probability of a mutant that appears proportionally to reproduction rate. Parameter values are the same as in Fig. 1B, and F and B are initially at steady state as in Fig. 1B, while mutants are introduced locally (in practice at numerical integration time t = 500 h) by using the initial condition in Eq. 3, with a total number NM=3.33×10−11 of mutants introduced in the system. Credit: DOI: 10.1073/pnas.2108671119
Scientists at EPFL and Sorbonne propose a new model of the diversity and evolution of gut bacteria that shows how the gut environment helps neutral mutations become prevalent, with significant potential implications on health and metabolic diseases.
“We are used to thinking of evolution as a very slow process, and this is definitely the case for large mammals etc,” says Professor Anne-Florence Bitbol at EPFL’s School of Life Sciences. “But viruses evolve quite fast, as the quick succession of COVID-19 variants shows us. Bacteria evolve at intermediate timescales, and those present in our gut can evolve at timescales relevant for us.”
The mutation and evolution of gut bacteria, can affect public health in a significant way; an obvious example is antibiotic resistance. Consequently, much research has gone into working out the factors that influence the evolution of gut bacteria, and some studies have pointed to the actual structure of the gut, including its hydrodynamic flow that causes different gradients of food and bacterial concentrations across its length.
A new model of the gut
Working with Darka Labavić and Claude Loverdo of Sorbonne University, Bitbol has now developed a “minimal model” of bacterial mutation and evolution in the gut, providing some keen insights about how the guts internal flow affect the distribution of the gene pool. The study is published in PNAS.
“Our model demonstrates that the specific spatial structure of the gut, with hydrodynamics and concentration gradients, can substantially increase the probability that neutral mutants reach high proportions and eventually take over the population,” says Bitbol. These neutral mutants refer to bacteria that undergo mutations that have no effect on growth rate, or at least their impact is negligible in the context of an entire bacterial population.
“As the gut’s environment strongly increases the expected average proportion of neutral mutants, it also increases their ability to ultimately take over the population, compared to a standard well-mixed population,” says Bitbol. “Thus, the specific environment of the gut enhances neutral bacterial diversity.”
Neutral mutations in gut health and disease
But what does a prevalence of neutral mutants in the gut mean? “It means that a larger fraction of mutants that appear randomly can reach important fractions instead of rapidly dying out,” explains Bitbol. “This can increase the diversity of the gut microbiota as well as its ability to adapt to environment changes. And because the composition of the gut microbiota can impact metabolism, this could have indirect implications on metabolism.”
And the findings can also have implications on how we treat metabolic diseases, such as inflammatory bowel disease. “A key point is that the increase of diversity and of adaptability that we predict is strongly associated with the existence of strong gradients of food and bacterial concentrations in the colon,” says Bitbol. “Bacterial concentrations are much lower in the small intestine than the colon, so at the entrance of the colon, there is a lot of food and fewer bacteria.”
“But further down the intestine, bacteria have eaten the food and reproduced, so there is less food but more bacteria. If these gradients are weaker, then the enhancement of diversity that our model predicts will also weaken. This can happen if the flow velocity in the gut changes or if the contractions of the gut muscles become stronger or weaker. So there might be possible links to metabolic disorders such as inflammatory bowel disease. Understanding these effects could potentially be a first step toward learning to alter them to improve such conditions.”
Next steps
Bitbol is already planning to follow this study up to answer other questions. “So far, we have worked on neutral mutants for simplicity, but investigating the case of beneficial mutants—those that increase growth rate—and deleterious mutants—those that decrease growth rate—would be extremely interesting and would give a more complete picture of the impact of the spatial structure of the gut on the evolution of gut bacteria. There is also a lot to do to gradually increase the realism of the model.”

Leave a Reply