As researchers mark the loss of the first living recipient of a pig kidney, they share what they’ve learnt about xenotransplantation.
By Max Kozlov
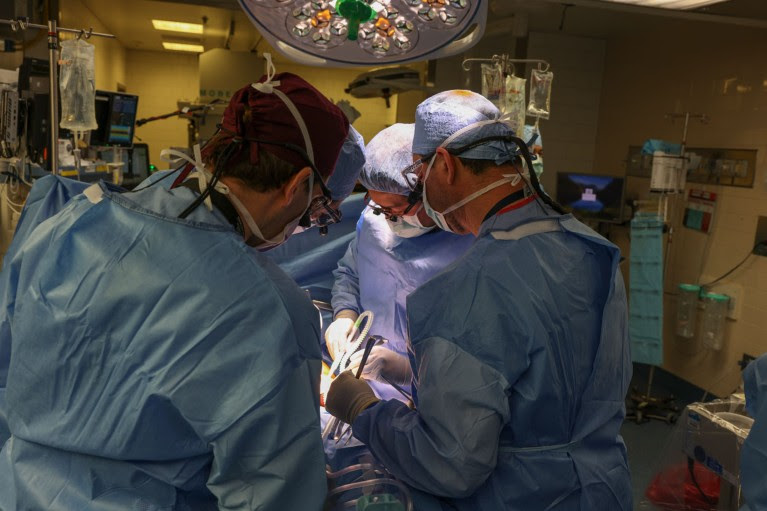
Surgeons transplant a pig kidney into 62-year-old Richard Slayman, who died this month of causes unrelated to the procedure.Credit: Massachusetts General Hospital
Last week, the first living person to receive a kidney from a pig died, just under two months after his transplant — sooner than his doctors had expected. But the timing is in keeping with that of the first people to receive pig hearts, both of whom died around two months after their transplants.
The relatively short survival time for all three recipients demonstrates that these pioneering cross-species transplants “have not had as great success as would have been predicted from the primate studies”, says Robert Montgomery, a transplant surgeon at New York University (NYU) in New York City.
But the three procedures offered hope to desperately ill people who had run out of options. And researchers say that they have learnt valuable lessons from the first pig-organ transplants into humans, on topics ranging from the types of medication that recipients need to the amount of testing that pig organs must undergo. “This is not an insolvable problem,” Montgomery says. “I’m encouraged that we’re as far along as we are.”
Nature spoke to xenotransplant surgeons about what they’ve learnt so far, and how they see the field moving forwards.
Relieving a shortage
The use of organs from other species in humans, called xenotransplantation, has long been a dream of surgeons because of the chronic shortage of suitable human organs. Researchers have homed in on pigs as a donor species, in part because their organs’ size and anatomy resemble those of humans.
Data from non-human primates that have received pig organs are promising: a study1 published in 2023 reported that five monkeys each survived for more than one year after receiving transplanted pig kidneys.
First pig liver transplanted into a person lasts for 10 days
The first xenotransplant into a living person was in 2022, when 57-year-old David Bennett received a pig heart and survived for 60 days after the procedure. A second man, Lawrence Faucette, received a pig heart in 2023 and survived for 40 days.
Muhammad Mohiuddin, a surgeon at the University of Maryland School of Medicine in Baltimore who was on the care team for both pig-heart transplants, cites several possible explanations for Bennett’s death. In the weeks before he died, Bennett had an infection, so physicians gave him an immune-boosting therapy made up of pooled antibodies from thousands of donors. Scientists later found that some of the antibodies had reacted to the pig organ2, meaning that the treatment could have exacerbated Bennett’s condition. Since then, Mohiuddin has worked with local blood banks to develop ways to screen for reactive antibodies.
Another possible explanation for Bennett’s limited survival is a latent infection of the transplanted heart with a pathogen called porcine cytomegalovirus, which might have been activated and then harmed the heart. The virus was found in the organ after Bennett’s death but was missed by tests before the transplant, signalling that more sensitive tests must be used to screen organs, Mohiuddin says.
Compassionate use
All the xenotransplants into living people have received ‘compassionate use’ approval from the US Food and Drug Administration (FDA), granted in rare cases in which a person’s life is at risk and there are no other treatments available. People treated on such grounds tend to be much sicker than the average person on the transplant waiting list, making it difficult to work out whether an unfavourable outcome is the result of the procedure or the recipient’s poor health, Mohiuddin says. That’s why some researchers have been pushing for the FDA to begin clinical trials of the procedure, which would allow for systematic evaluation of its performance.
It’s possible, for example, that poor underlying health contributed to the death on 7 May of Richard Slayman, the first living recipient of a pig kidney. Tatsuo Kawai, one of the surgeons who conducted the transplant at Massachusetts General Hospital in Boston, tells Nature that Slayman’s kidney was functioning well the day before his death and that he died for reasons unrelated to his transplant. In the year before the procedure, Slayman had developed congestive heart failure.
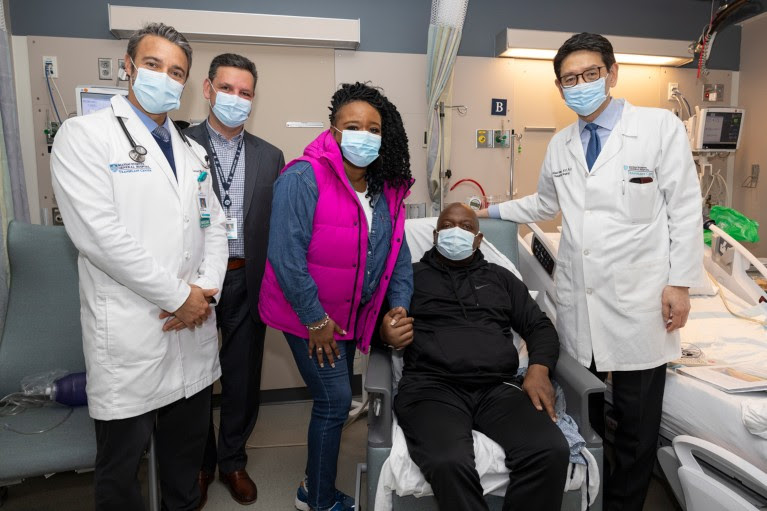
Pig-kidney recipient Richard Slayman (seated) with his partner and doctors.Credit: Michelle Rose/Massachusetts General Hospital
Researchers are also experimenting with what can be done before the transplant to best prevent organ rejection. One technique is genetically modifying the donor pigs, but the number of genetic edits necessary to stave off rejection is far from settled, Montgomery says.
eGenesis, a biotechnology firm in Cambridge, Massachusetts, that bred the pig used in Slayman’s surgery, has produced pigs with a record 69 edits, both to avoid rejection and to reduce the risk that a virus lurking in the organ could infect the recipient. Meanwhile, Revivicor, a firm in Blacksburg, Virginia, has opted for about ten genetic edits.

Pig organs partially revived in dead animals — researchers are stunned
In the fourth and latest xenotransplant in a live person, Montgomery and his team tried a new approach using the thymus, an immune-related organ that could help teach the recipient’s immune system to recognize the pig organ. They grafted the source pig’s thymus to the kidney and then transplanted both into 54-year-old Lisa Pisano on 12 April. They used a pig with only a single genetic modification, which could make scaling up the production of pig organs easier, Montgomery says. Pisano remains in stable condition in hospital, he adds.
There is still much more to be learnt, he says. In a forthcoming study and in one published today in Nature Medicine3, Montgomery and his colleagues analysed tissue samples from two people who had been declared legally dead before receiving a pig heart and found that at the cellular level, rejection of xenotransplanted organs looks “very different” from that of organs transplanted from a human donor, Montgomery says. He adds that these findings could help researchers to anticipate rejection and develop tailored immunosuppressant regimens for future surgery.

Leave a Reply