by Emily Moskal, Stanford University
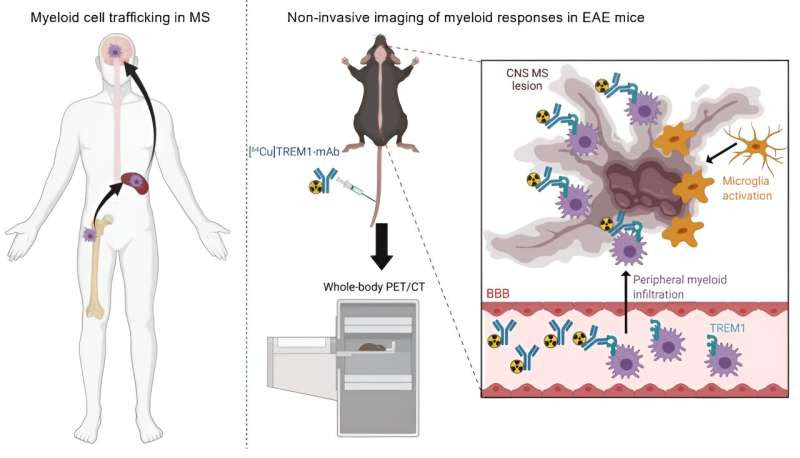
Imaging approach for the detection of activated peripheral CNS-infiltrating myeloid cells. Peripheral myeloid cells (monocytes, macrophages, neutrophils, and dendritic cells) are recruited to the central nervous system (CNS) during multiple sclerosis (MS). Peripheral myeloid cells, in addition to brain resident microglia, are associated with CNS MS lesions and are fundamental to disease progression and remission. We have identified triggering receptor on myeloid cells 1 (TREM1) positron emission tomography (PET) as a new approach to detect pathogenic peripheral CNS-infiltrating myeloid cells in the experimental autoimmune encephalomyelitis (EAE) mouse using [64Cu]TREM1-mAb whole-body PET/CT. Credit: Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abm6267
By the time multiple sclerosis is normally diagnosed—when brain lesions appear on magnetic resonance imaging scans—the patient can have irreversible damage and treatment options can be limited. But in a Stanford Medicine-led study, researchers found a molecular signal that could help confirm diagnosis of multiple sclerosis early and dramatically improve treatment through sensitive, real-time monitoring of patients’ therapeutic response.
Multiple sclerosis is a disease in which the immune system attacks myelin sheath—the protective covering of nerve cells—causing communication problems between the brain and the rest of the body. The disease affects nearly a million people nationally.
The most definitive signs of multiple sclerosis are devastating brain lesions, areas of damaged brain tissue that cause a variety of symptoms including difficulty with speech and movement. Other symptoms, such as fatigue and loss of balance, occur earlier on in disease progression, but they overlap with other diseases, muddying doctors’ ability to clearly diagnose the ailment. To complicate matters further, the most common method of diagnosis, MRI, only reveals the disease once there’s already damage to the brain.
“Unfortunately MRI is not sensitive enough to image the earliest stages of disease. This is because it mainly detects structural or functional changes that occur later, as opposed to biochemical alterations that typically occur before significant damage to the brain,” said Michelle James, Ph.D., the senior author of the study and assistant professor of radiology and neurology.
Scientists think that detecting certain biochemical interactions in the body could help doctors make a diagnosis before symptoms worsen. One such signal is that of harmful inflammation, however the currently available imaging techniques have a severe disadvantage: They don’t distinguish between good (like when immune cells flock to the site of a cut to help heal and fight possible infection) versus bad inflammation.
Because bad inflammation causes damage to myelin sheaths in multiple sclerosis, scientists want to track the innate immune system, which spurs the first inflammatory response to foreign invaders, so they are better able to pinpoint onset and progression of the disease, said James.
“The tools that have been around for the past couple of decades were not specific to just the innate immune cells,” said James, who’s also a member of the department of radiology’s molecular imaging program. They also tracked macrophage cells, a type of white blood cell that digests bad microorganisms early on in inflammation when the molecules they tracked are expressed.
In a study led by James and published June 28 in Science Translational Medicine, scientists found a protein that does discern between the two inflammatory variants, lighting up on positron emitting tomography, which uses radioactive tracers or tags to highlight the proteins of interest, when the first signs of inflammation driven by the innate immune system are activated in mice with multiple sclerosis. Researchers tracked the receptor known as TREM1, found on the surface of innate immune cells.
Scientists previously knew that TREM1 plays a role in amplifying inflammation, but they didn’t know exactly where in the body or in which disease-related context. The study showed that TREM1 PET imaging has 14 to 17 times the sensitivity to detect bad neuroinflammation than established imaging techniques.
“The mice looked completely normal, but we saw this blazing signal where cellular events were already occurring, creating damage,” James said. “With this new non-invasive imaging approach, we can detect toxic inflammation that could help us better understand and treat diseases.”
Aisling Chaney, Ph.D., a former postdoctoral scholar in James’ lab who is now an assistant professor at Washington University in St. Louis, is lead author of the paper.
Painting a picture of multiple sclerosis
To better understand the early contributors to inflammation in multiple sclerosis, researchers put a tag on an antibody that targets TREM1 and imaged its presence in mice. In the study of 178 mice, researchers saw that TREM1 lit up on PET scans only for mice with multiple sclerosis, before and after symptoms appeared. Scientists scanned the torsos, spinal cords, brains, spleens, femurs and heart in a whole-body scan of mice with and without multiple sclerosis.
By tracking TREM1’s location they found that immune cells from outside the central nervous system had infiltrated the brain and spinal cord, leading to significant damage because the cells were not normally there. The researchers also used mice with the gene for TREM1 inactivated to confirm its role in inflammation. Inflammation went away when the gene was knocked out.
“Once you knock out that gene or reduce TREM1 signaling pharmacologically, you’re preventing the crazy wildfire that is inflammation,” James said. “And the good thing is you’re dampening down the maladaptive or toxic inflammation without affecting beneficial inflammation.”
While the researchers focused on mouse models in this study, scientists also used human brain tissue affected by multiple sclerosis to search for TREM1 activation, which they confirmed was present.
Disease monitoring
Given the specificity of TREM1’s link to bad inflammation in the brain, James and her team wanted to know whether it could be used as an indicator of therapeutic response. In the study, scientists observed that administration of the FDA-approved therapy siponimod, a drug that reduces the reappearance of multiple sclerosis symptoms by decreasing immune cell activation, reduced TREM1 activity in mice. None of the mice treated with it demonstrated symptoms of multiple sclerosis.
The reduction in TREM1 activity and symptom severity suggest that the molecule contributes to disease progression when present and is a potential therapeutic target to reduce symptoms, said James.
By targeting TREM1 therapeutically and having a diagnostic to select patients and monitor their response to these drugs early, patients won’t need to wait for the usual 12-month follow up (in which time, they could have significant damage to the brain) but can instead see which therapy is working for them as soon as possible.
“Experimenting with different drugs rather than targeting known molecular changes is currently a trial-and-error process, which is like playing dice with your brain,” James said.
Because MRIs are readily available and cheaper than PET, they will always be the first line of action, James said. If MRI results rule out a tumor and other neurological conditions, but are suggestive of multiple sclerosis, a TREM1 PET scan could quickly confirm the early diagnosis and enable a rapid start to therapy and its monitoring.
James anticipates that this technique has the potential to be translated to the clinic with broad application within three to five years.
This technique could be applied to other diseases, as harmful inflammation is a common driver of many diseases, and imaging TREM1 can tell scientists the stage of disease and help scientists monitor response to drugs. For instance, James and her team have shown that TREM1 can detect inflammation in mouse models of stroke and Parkinson’s disease, and are exploring models of Alzheimer’s disease and brain tumors.
“Toxic TREM1 activation could detect a number of diseases because it is a common process that’s driving pathology in a ton of diseases, including COVID-19, inflammatory bowel disease, arthritis and Alzheimer’s,” James said.

Leave a Reply