August 30, 2024
by Centro Nacional de Investigaciones Cardiovasculares Carlos III (F.S.P.)
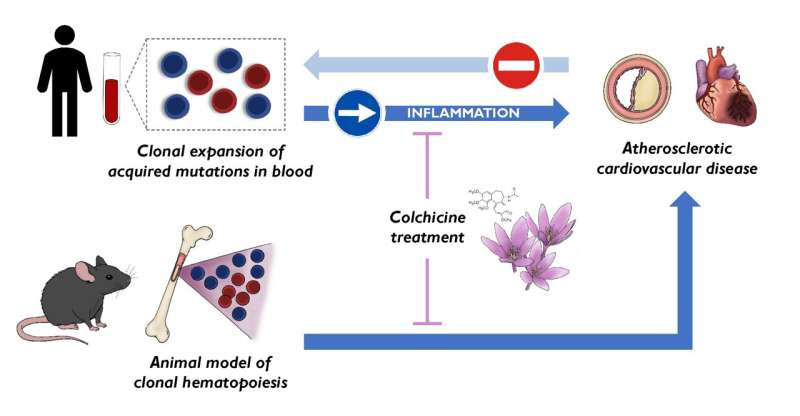
Summary of main findings by CNIC researchers. Clonal hematopoiesis and the expansion of blood cells carrying certain acquired mutations represent a new cause of atherosclerosis and cardiovascular disease, not a consequence of it. The effects of some of these mutations can be attenuated by a plant-derived anti-inflammatory drug, colchicine, laying the groundwork for possible personalized medicine strategies. Credit: CNIC
To the known risk factors for cardiovascular disease—high blood pressure, high cholesterol, diabetes, overweight and obesity, smoking, and physical inactivity—a new one has to be added, clonal hematopoiesis. This condition is triggered by acquired mutations in blood stem cells and was already known to be associated with an elevated cardiovascular risk.
However, until now it was uncertain if clonal hematopoiesis was a cause or consequence of cardiovascular disease.
Now, a new study published in Nature Medicine and carried out by researchers at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) resolves this critical debate by establishing clonal hematopoiesis as a new risk factor for atherosclerosis—the formation of lesions in the arterial wall that underlies most cardiovascular disorders.
In a second study, published in the European Heart Journal, the CNIC scientists propose the ancient medication colchicine as the central plank of personalized strategies to alleviate the effects of clonal hematopoiesis associated with acquired mutations in the TET2 gene. The results of these important studies were presented at the European Society of Cardiology meeting in London, UK.
Acquired mutations in blood cell lineages: A new cause of atherosclerosis
An adult person produces hundreds of thousands of blood cells every day. This high rate of cell division unavoidably entails the accumulation of DNA mutations in the dividing cells. These mutations are known as somatic mutations, and are acquired, not inherited.
“Although most somatic mutations are innocuous, some give the affected cells a competitive advantage that allows them to expand and progressively accumulate, generating clonal populations of mutated blood cells, a phenomenon known as clonal hematopoiesis,” explained José Javier Fuster, who led the Nature Medicine study.
These mutations had already been proposed as a possible risk factor for cardiovascular disease; however, the exact nature of the relationship had remained unclear.
As Dr. José Javier Fuster, coordinator of the CNIC “Novel Mechanisms of Atherosclerosis” program, explained, “some earlier studies suggested that somatic mutations linked to clonal hematopoiesis contribute directly to cardiovascular disease and thereby accelerating the development of atherosclerosis.
“On the other hand, others proposed that it is atherosclerosis that causes clonal hematopoiesis by increasing the proliferation of blood stem cells and thereby generating a higher proportion of mutated blood cells.”
The Nature Medicine study clarifies the relationship between clonal hematopoiesis and atherosclerosis through a longitudinal analysis of data from the PESA-CNIC-Santander study. PESA (Progression of Early Subclinical Atherosclerosis) is a prospective study of more than 4000 apparently healthy middle-aged participants who have undergone periodic examinations using advanced imaging technology since 2010 to detect the presence and progression of asymptomatic atherosclerosis. PESA is a collaborative initiative of the CNIC and Santander Bank.
“The PESA study has already made very important contributions to our understanding of cardiovascular disease, and its longitudinal nature and unique characteristics provide an ideal framework for carrying out this important study on the relationship between clonal hematopoiesis and atherosclerosis,” said Dr. Valentín Fuster, CNIC General Director, principal investigator on the PESA study, and co-lead author on the Nature Medicine study.
The researchers used high-sensitivity DNA sequencing technology to detect somatic mutations in blood samples and assessed the presence and progression of atherosclerosis detected with noninvasive imaging techniques in the PESA participants.
“The study was a multidisciplinary effort involving specialists in basic science and cardiology, together with the specialized technical expertise of the Bioinformatics, Genomics, and Clinical Trials Units at the CNIC,” said José Javier Fuster.
The results of the study clearly demonstrate that participants who had mutations linked to clonal hematopoiesis at the start of the study were more likely to develop atherosclerosis in the following years. On the other hand, the presence and extent of atherosclerosis had no influence on the expansion of mutated blood cells.
“These results indicate that the mutations contribute to the development of atherosclerosis but are not a consequence of it,” explained co-first author Miriam Díez-Díez.
“However, it remains possible that other factors, such as genetic profile or lifestyle, might modulate the effects of clonal hematopoiesis, and future studies are planned to examine this possibility,” added Beatriz L. Ramos-Neble, the other co-first author on the study.
The results of the study have clear clinical implications and identify clonal hematopoiesis as a cardiovascular risk factor completely different from the traditional risk factors studied in recent decades. This novelty holds promise for the development of new strategies for the prevention of cardiovascular disorders.
“By demonstrating that the mutations linked to clonal hematopoiesis precede atherosclerosis and contribute to its development, our research suggests that blocking the effects of these somatic mutations could help to prevent cardiovascular disease,” claimed Dr. José Javier Fuster. The second CNIC study, published in the European Heart Journal, lays the groundwork for this.
An ancient drug to alleviate a new cardiovascular risk factor
The best characterized mutations linked to clonal hematopoiesis are those that affect the TET2 gene. In a 2017 study published in Science, Dr. José Javier Fuster’s team showed that mutations in TET2 accelerate the development of atherosclerosis in animal models.
In the new study published in the European Heart Journal, the CNIC group, in partnership with the team led by Dr. Pradeep Natarajan at the Broad Institute in Boston, show that the adverse effects of TET2 mutations on cardiovascular health can be alleviated by treatment with the anti-inflammatory drug colchicine.
The CNIC team demonstrated that administration of colchicine to animals with TET2 mutations slows the development of atherosclerosis to a rate similar to that seen in non-mutated animals. In parallel, the Broad Institute scientists showed that individuals with TET2 mutations and who had been treated with colchicine for other conditions had a lower risk of myocardial infarction than untreated patients with similar mutations.
Plant preparations containing colchicine have been used for thousands of years in traditional medicine, and the drug is used in modern medicine to treat inflammatory conditions such as gout.
“Colchicine is a very cheap medicine, available throughout the world, and is approved for the prevention of cardiovascular disease by the European Medicines Agency and by the FDA in the U.S.. There is therefore no major obstacle to its use for the prevention of cardiovascular disease in people with TET2 mutations,” emphasized Dr. María Ángeles Zuriaga, who conducted the experimental studies at the CNIC and is the first author on the European Heart Journal study.
Dr. José Javier Fuster underlined the important implications of the study for personalized medicine.
“In clonal hematopoiesis, each mutated gene acts through different mechanisms and will therefore likely require specific interventions to target its effects. This study lays the groundwork for using colchicine for/in the personalized prevention of cardiovascular disease of carriers of mutations in TET2, but new clinical trials will be needed to conclusively demonstrate its effectiveness in these individuals.”
More information: Unidirectional association of clonal hematopoiesis with atherosclerosis development, Nature Medicine (2024). DOI: 10.1038/s41591-024-03213-1
M A Zuriaga et al, Colchicine prevents accelerated atherosclerosis development in TET2-mutant clonal hematopoiesis, European Heart Journal (2023). DOI: 10.1093/eurheartj/ehad655.3264
Journal information: European Heart Journal , Science , Nature Medicine
Provided by Centro Nacional de Investigaciones Cardiovasculares Carlos III (F.S.P.)

Leave a Reply