September 11, 2024
by Kendall Daniels, University of North Carolina at Chapel Hill School of Medicine
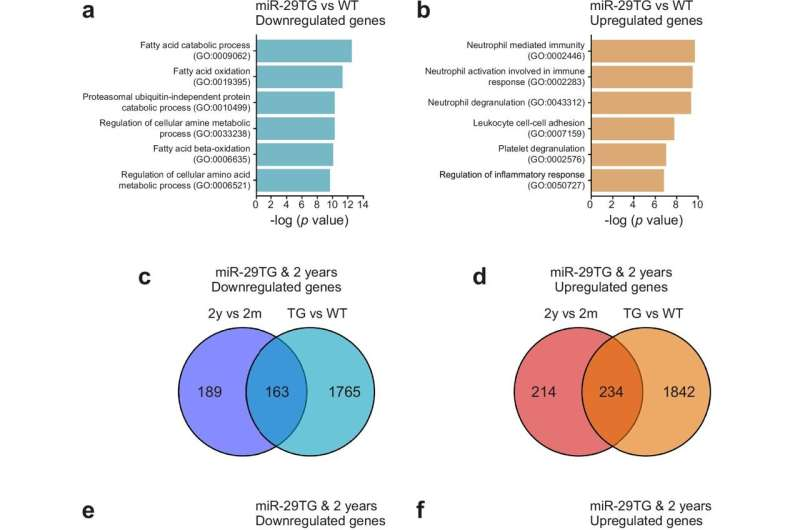
The liver of miR-29TG and 2 year-old wildtype mice share changes in key aging-related genes and pathways. Credit: Communications Biology (2024). DOI: 10.1038/s42003-024-06735-z
A team led by UNC School of Medicine researchers is the first to confirm that a small molecule called miR-29 plays a role in driving aging. Their new findings are published in Communications Biology.
MicroRNAs are small, single-stranded nucleic acids found in cells and in the bloodstream. These specialized molecules play an important role in health and disease, as they are tasked with making sure that genetic products, such as RNA and proteins, are produced at exactly the right amounts needed in the body.
The researchers were led by Mohanish Deshmukh, Ph.D., professor in the Department of Cell Biology and Physiology and the UNC Neuroscience Center, and Praveen Sethupathy, Ph.D., a former professor in the Department of Genetics and now chair of Biomedical Sciences at Cornell University.
The Deshmukh lab has long studied a specific microRNA called miR-29. Previous research has found that the molecule plays a critical role in maintaining brain and heart function. MiR-29 levels were also known to increase with age in multiple tissues, but it was unclear if this molecule directly contributes to aging or if the increase in levels over time was merely coincidental.
So, in collaboration with Norman E. Sharpless, MD, professor of cancer policy and innovation at UNC Lineberger Comprehensive Cancer Center and a prominent expert in aging, the Deshmukh and Sethupathy labs embarked on a research mission to understand if miR-29 plays a direct role in driving the natural process of aging.
Deshmukh and Sethupathy, who are both co-senior authors on the study, performed an unbiased computational analysis. With thousands of microRNAs performing multitudes of roles in the body, they wanted to be sure that they were homing in on the exact microRNA that is responsible for aging-related changes in gene expression.
Small RNA molecule has role in driving agingmiR-29 is a candidate driver of gene expression changes in aged mice. Credit: Communications Biology (2024). DOI: 10.1038/s42003-024-06735-z
After combining all potential microRNAs and genetic alterations, their approach determined that miR-29 ranked highly among the molecules most likely to influence the aging-related changes in gene expression.
Next, researchers conducted animal model experiments to address a simple question: can aging be slowed down with less miR-29 in the body and can aging be accelerated with more miR-29? Using a mouse model of progeria, a genetic disorder that causes rapid aging, researchers found that reducing the levels of miR-29 significantly extends the lifespan of the progeria mouse model.
Researchers then tested the other side of the coin by increasing the levels of miR-29 in healthy mouse models. Within two months, the mouse model exhibited the striking symptoms of aging very early on in their lives.
With miR-29’s functional role in aging now confirmed, researchers have more questions regarding the specific tissues in which miR-29 is important and how researchers can reduce miR-29 in some tissues and not others.
One of the lab’s future endeavors is to find out if miR-29 needs to be present in a specific organ in the body, or multiple, to drive the aging process. Since the mouse models for the study were created to express the molecule in every tissue, researchers are curious to know if expressing this solely in any one tissue is sufficient to drive aging or if miR-29 is really needed in every tissue to effectively drive aging.
The labs are also interested in selectively inhibiting miR-29, to see if it’s feasible to delay normal aging. However, doing so is easier said than done. Because this microRNA has important roles in the brain and the heart, researchers will need to be careful in not disrupting miR-29 activity in those specific tissues.
More information: Vijay Swahari et al, miR-29 is an important driver of aging-related phenotypes, Communications Biology (2024). DOI: 10.1038/s42003-024-06735-z
Journal information: Communications Biology
Provided by University of North Carolina at Chapel Hill School of Medicine

Leave a Reply