July 25, 2024
by The University of Hong Kong
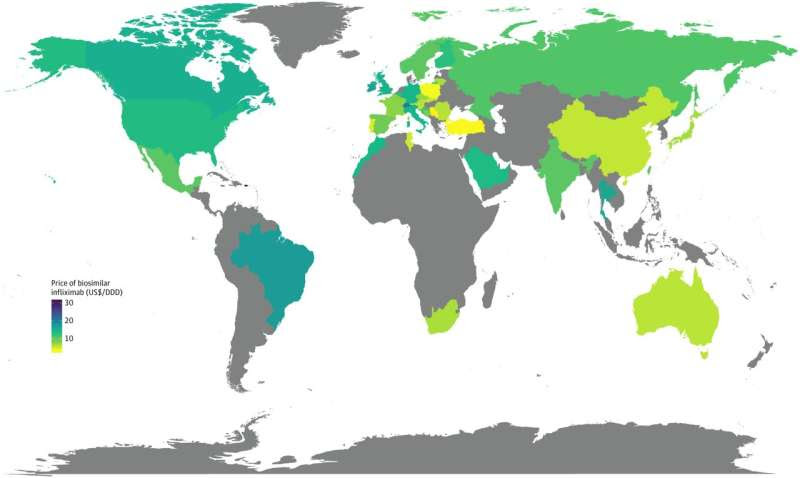
Global price of biosimilar infliximab in July 2022. Credit: JAMA Network Open (2024). DOI: 10.1001/jamanetworkopen.2024.18800
A research team from the LKS Faculty of Medicine of the University of Hong Kong (HKUMed) performed a cost-effectiveness analysis of treatment strategies for patients in Hong Kong with rheumatoid arthritis.
The researchers found that for patients who had an inadequate response to the first-line therapy of methotrexate, using biosimilar disease-modifying anti-rheumatic drugs (DMARDs) is more cost-effective than the commonly used leflunomide.
By adopting biosimilars, health care institutions in Hong Kong can optimize resource allocation, improve overall patient outcomes, and establish a more efficient and sustainable health service model. The study findings were published in JAMA Network Open.
Rheumatoid arthritis (RA) is a prevalent chronic inflammatory disorder impacting patients’ joints and overall quality of life. Methotrexate is the standard first-line therapy, but it fails in over half of patients.
Clinical guidelines recommend adding or switching to other conventional synthetic DMARDs (csDMARDs), such as leflunomide, after methotrexate failure in patients without additional risk factors. Recent evidence suggests that the biologic DMARDs (bDMARDs) present a better treatment response and retention rate than csDMARDs in both clinical trials and real-world practice.
However, the relatively high cost of biologic originators limits their use. Emerging biosimilar DMARDs (biosimilar adalimumab and biosimilar infliximab) have comparable efficacy and safety to their reference biologics and reduce cost by more than half in Hong Kong, allowing patients to access the optimal treatment earlier.
The research team retrospectively analyzed data from the local RA cohort of 25,099 patients in total, complemented with data from landmark trials, and developed a disease simulation model to compare the lifetime health care costs and quality-adjusted life years (QALYs) for three different treatment regimens: biosimilar infliximab (HK$1,185,096, QALYs:15.35); biosimilar adalimumab (HK$ 1,131,360, QALYs:15.55); and leflunomide (HK$1,203,037, QALYs:14.82).
The analysis suggested that biosimilar treatment strategies, particularly biosimilar adalimumab, appeared to be the most cost-effective, with lower health care costs and greater QALY improvement than leflunomide.
The projected budget savings were HK$17,941 and HK$71,677 per patient treated by biosimilar infliximab and biosimilar adalimumab, respectively. The price of biosimilar DMARDs in Hong Kong falls within the mid-range (ranked 14th among 29 high-income countries/regions) in the global context.
According to the latest Hospital Authority Drug Formulary of Hong Kong, bDMARDs are currently listed as self-financed items with a safety net. Patients must fulfill financial and clinical eligibility criteria for the Samaritan Fund to buy bDMARDs at subsidized prices. Otherwise, they have to pay the full cost themselves.
Nearby Asian countries and regions such as Japan, Korea and Taiwan have already included at least one bDMARD in their public formularies, indicating the potential for Hong Kong to refine its health care policies and expedite the addition of these treatments to the public formulary.
“Adopting biosimilars improves patients’ quality of life and reduces medical costs from the perspective of public health care in Hong Kong,” said Professor Shirley Li Xue, who led the research study.
“Our study is a good attempt to apply ‘health economics’ to inform decision-making in drug introduction and formulary listing. This systematic evaluation process helps decision-makers assess the efficacy, safety and cost-effectiveness of new treatments by providing transparent, evidence-based insights.”
“Efficient value-based assessment mechanisms can significantly reduce drug approval and formulary listing timelines,” Professor Li added, ‘which is crucial for ensuing stakeholder engagement and optimizing resource allocation in the health care system.”
More information: Kuan Peng et al, Cost-Effectiveness of Biosimilars vs Leflunomide in Patients With Rheumatoid Arthritis, JAMA Network Open (2024). DOI: 10.1001/jamanetworkopen.2024.18800
Journal information: JAMA Network Open
Provided by The University of Hong Kong

Leave a Reply