by Olivia Dimmer, Northwestern University
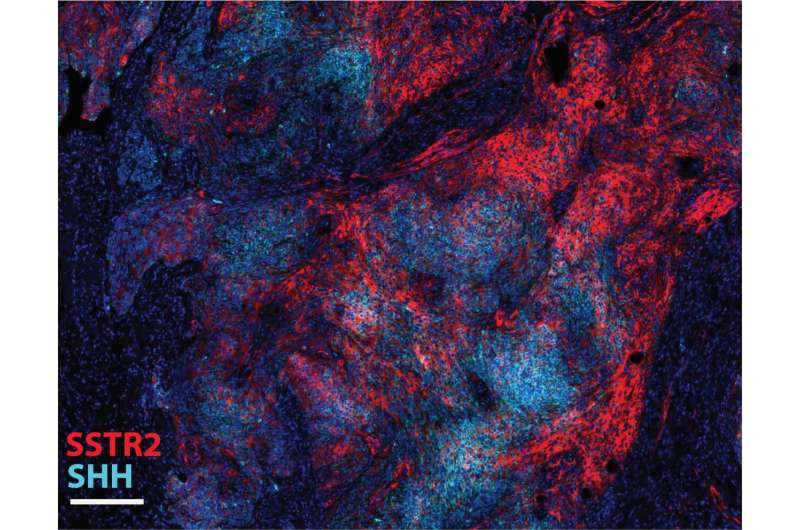
Multiplex immunofluorescence staining and imaging using the COMET system platform on a human meningioma patient sample, showing SSTR2+ tumor cells (in red), expressing the hedgehog pathway activation marker sonic hedgehog (SHH, in cyan blue). Credit: Hinda Najem
Scientists have uncovered a genetic explanation for one subset of common brain tumors, according to a study published in Nature Communications.
Meningiomas—tumors that begin around the brain or spinal cord—are the most common type of tumors found in the central nervous system. An estimated 25,000 Americans are diagnosed with meningiomas each year, according to the National Institutes of Health.
Although many meningiomas can be treated with surgery, there are a substantial number of patients for whom the tumor can’t be fully removed and continues to grow. Although more aggressive meningiomas may be treated with radiation with variable results, there are currently no chemotherapies available.
Previous studies have established genetic causes in approximately 80% of meningiomas, however the molecular drivers of the remaining were not well understood, said Mark Youngblood, MD, Ph.D., a current fifth-year Northwestern Medicine Neurosurgery resident and first author of the study. This led Youngblood to investigate the genetic drivers of the tumors as part of his Ph.D. thesis while at the Yale School of Medicine.
In the study, Youngblood and his collaborators performed integrated genomic analyses on tumor samples obtained from patients. They found that a subset of meningiomas exhibited structural variations—such as chromosomal breakage—that triggered upregulation in the hedgehog signaling pathway, a collection of genes known to be crucial for early development and cell differentiation. (The hedgehog signaling pathway was first identified in fruit flies and is named because flies lacking these genes were said to resemble hedgehogs.)
Investigators then took a closer look at the two genes that occurred near these structural breaks: IHH and SHH.
“When we looked at the RNA sequencing data, we found that hedgehog ligand genes IHH and SHH had dramatically increased expression compared to meningiomas that did not have these structural variants, explaining the activation of hedgehog signaling in these cases,” Youngblood said.
To understand how those structural variants lead to increased gene expression, Youngblood and his collaborators employed Hi-ChIP genomic analysis, which allowed them to analyze the DNA in three dimensions. They found that the structural variants created a new interaction between chromosomal regions that normally do not meet, which then increased the expression of IHH.
The findings of the study offer a genetic explanation for a subset of meningiomas which did not previously have a well-defined cause, Youngblood said.
“We’ve explained the genomic drivers behind about a third of these meningiomas that previously had no drivers. We’ve also expanded the role of hedgehog signaling in meningiomas. This is the first time that enhancer hijacking of hedgehog pathway ligands has been demonstrated in any form of cancer, which is interesting because this particular pathway plays such a large role across different types of cancers.”
FDA-approved drugs targeting the hedgehog signaling pathway already exist and, after further research and clinical trials, could be a potential treatment path for patients with meningiomas that express this pathway, said Amy Heimberger, MD, Ph.D., the Jean Malnati Miller Professor of Brain Tumor Research, vice chair for Research in the Department of Neurological Surgery and a co-author of the study.
“Number one, this study provides us with a potential target for patients that have the hedgehog pathway activated, which may be responsive to drugs that block this pathway,” said Heimberger, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “Number two, it provides a nice illustration of the heterogeneity among grade I meningiomas when we had previously presumed they are all the same.”
More information: Mark W. Youngblood et al, Super-enhancer hijacking drives ectopic expression of hedgehog pathway ligands in meningiomas, Nature Communications (2023). DOI: 10.1038/s41467-023-41926-y
Journal information: Nature Communications
Provided by Northwestern University

Leave a Reply