Content by Owlstone Medical Ltd
Reviewed by Louis Castel
The gut-liver axis is the term used to describe the two-way relationship between the gut microbiome and the liver. This relationship stems from the integration of signals produced by various factors such as diet, genetics, and the environment.
The liver and the gastrointestinal tract are connected by the portal vein, which allows the movement of gut-derived products through the blood to the liver. The liver secretes bile and antibodies into the gut, generating a feedback system known as the gut-liver axis.1Therefore, the gut microbiome plays an important role in the pathophysiology of many liver diseases.
The liver is the organ in closest contact with the intestinal tract and is exposed to many bacterial components and metabolites.2
Liver diseases like alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) have been linked to changes in the microbiome, a state referred to as dysbiosis. This dysbiosis can impact inflammation, fibrosis, and hepatic steatosis levels by interacting with the immune system and various cell types.2
The gut microbiome is made up of trillions of different microorganisms, such as bacteria, viruses, and fungi, which live in the gastrointestinal tract. These microorganisms have a significant impact on the pathological and physiological processes of their host – there is a mutual relationship resulting in many different roles in the body, such as maintaining a normal immune system and nutrient digestion and absorption.3
The significant role of the gut microbiome in liver disease is supported by mounting evidence indicating that several complications, including hepatic encephalopathy, can be effectively treated with prebiotics, probiotics, and antibiotics.4
A deeper understanding of the gut microbiome and its impact on liver diseases could offer clarity on these intricate disorders and pave the way for pioneering therapeutic strategies.
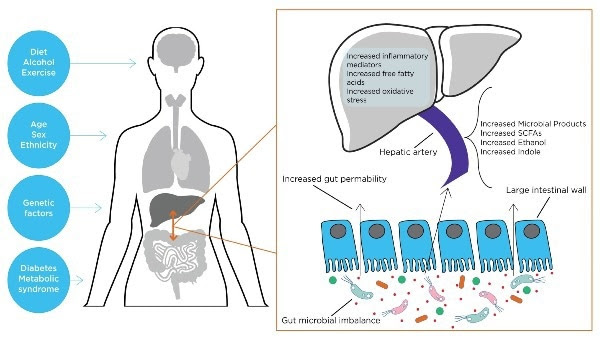
Image Credit: Owlstone Medical Ltd
Non-alcoholic fatty liver disease
NAFLD, a subtype of liver disease, is strongly associated with obesity, type 2 diabetes mellitus (T2DM), insulin resistance, hypertension, and metabolic syndrome. Within NAFLD, non-alcoholic steatohepatitis (NASH) represents a significant subtype that can progress to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). 5
NAFLD ranks among the most prevalent forms of chronic liver disease in the general population, with projections indicating a tripling of end-stage liver disease burden due to NAFLD by 2030. Typically associated with excessive adiposity, NAFLD correlates with a less diverse gut microbiome in overweight individuals compared to their lean counterparts. 5
Patients exhibiting NASH have a reduced proportion of Bacteroidetes compared to healthy controls, and patients exhibiting NAFLD have a heightened proportion of Escherichia, Anaerobacter, Lactobacillus, and Clostridium compared to healthy controls.6,7
Microbe products like endotoxins can become translocated in the portal circulation, leading to cytokine activation and interleukin release, which stimulates an inflammatory reaction, potentially resulting in hepatic inflammation and fibrosis.3
There are many different volatile organic compounds (VOCs) that can be detected in breath samples, providing insights into the mechanisms underlying NAFLD (Table 1). Additionally, microbial fermentation processes can yield ethanol, which in turn can promote the growth of gram-negative bacteria and increase intestinal permeability, thereby facilitating the absorption of inflammatory mediators.
Table 1. Volatile organic compounds (VOCs) are produced by many different mechanisms in NAFLD.8 Source: Owlstone Medical Ltd
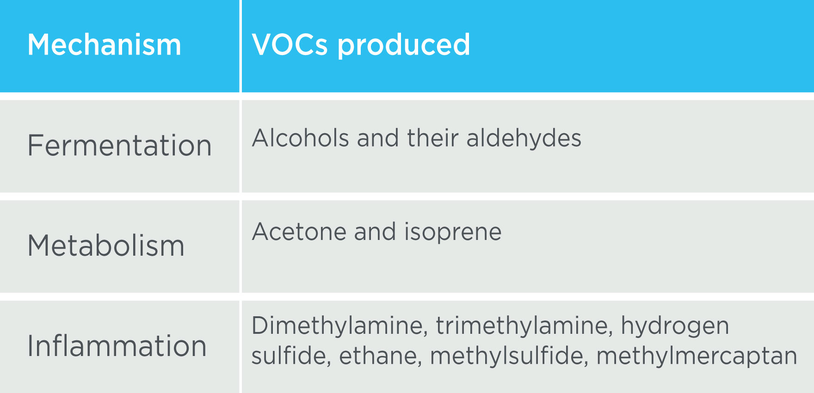
Volatile organic compounds (VOCs) are produced by many different mechanisms in NAFLD
Ethanol and liver disease
The gut microbiome is believed to have numerous implications in the development of non-alcoholic liver diseases. Patients diagnosed with NASH often exhibit elevated levels of ethanol-producing bacteria, such as Escherichia, and increased levels of circulating microbial ethanol, which is thought to be a result of functional impairment of hepatic insulin-dependent alcohol dehydrogenase (ADH). 9
When continuously created in relevant levels, microbially produced ethanol could potentially alter glucose and lipid metabolism and induce steatosis and liver inflammation.10
In healthy individuals, the liver metabolizes ethanol through the ADH and cytochrome P450 pathways, resulting in low circulating levels of ethanol in the bloodstream. However, in patients with liver disease, particularly those with end-stage liver disease, microbial ethanol production can surpass the liver’s ability to eliminate ethanol from the portal circulation.10 Thus, ethanol remains in the bloodstream, allowing it to circulate throughout the body.
Ethanol is volatile and can be detected in breath
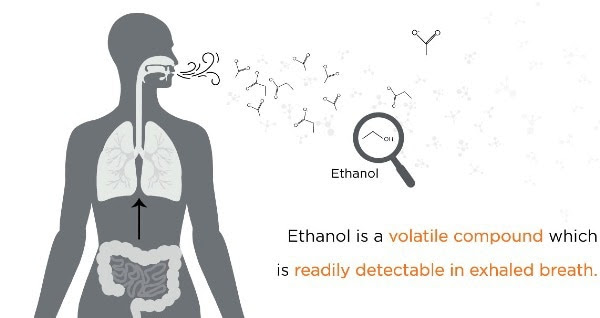
Image Credit: Owlstone Medical Ltd
Ethanol, being a volatile compound, is easily detectable in exhaled breath. Ethanol produced in the gut can enter the bloodstream, circulate throughout the body, and ultimately reach the air in the lungs through the network of blood vessels surrounding the alveolar membrane.
As a result, the air exhaled from the lungs is enriched with volatile compounds originating from various parts of the body, including the gastrointestinal tract. This means that ethanol detected in breath can serve as a window into ongoing microbial metabolism in the gut and may offer insights into the presence of liver disease.
While ethanol is one such volatile compound associated with liver-related microbial activity, numerous other biologically relevant VOCs in breath could also be linked to liver health.
Breath is essentially an inexhaustible resource, with large volumes that can be collected frequently, along with compounds that can be pre-concentrated before analysis. Due to this, breath analysis enables real-time longitudinal examination of volatile metabolites, with ethanol being a core biomarker for liver disease in clinical studies.
There are numerous other volatile compounds in Owlstone’s VOC Atlas, a catalog of identified VOCs often found on breath, several of which have been linked to liver disease.
Owlstone Medical’s Breath Biopsy OMNI service provides all the necessary resources for initiating breath analysis, along with guidance from a team of expert scientists. As a global leader in Breath Biopsy, Owlstone offers invaluable assistance to researchers looking to incorporate breath analysis into their studies.
References and further reading
Albillos A, Gottardi A de, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020 Mar 1;72(3):558–77. DOI: 10.1016/j.jhep.2019.10.003
Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016 Dec 1;65(12):2035–44. DOI: 10.1136/gutjnl-2016-312729
Albhaisi SAM, Bajaj JS, Sanyal AJ. Role of gut microbiota in liver disease. Am J Physiol-Gastrointest Liver Physiol. 2020 Jan;318(1):G84–98. DOI: 10.1152/ajpgi.00118.2019
Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017 Feb 23;2017(2):CD008716. DOI: 10.1002/14651858.CD008716.pub3
Younossi ZM. Non-alcoholic fatty liver disease – A global public health perspective. J Hepatol. 2019 Mar 1;70(3):531–44. DOI: 10.1016/j.jhep.2018.10.033
Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58(1):120–7. DOI: 10.1002/hep.26319
Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015 Feb 3;5(1):8096. DOI: 10.1038/srep08096
Solga SF. Breath volatile organic compounds for the gut-fatty liver axis: Promise, peril, and path forward. World J Gastroenterol WJG. 2014 Jul 21;20(27):9017–25. DOI: 10.3748/wjg.v20.i27.9017
Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. DOI: 10.1002/hep.26093
Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022 Oct;28(10):2100–6. DOI: 10.1038/s41591-022-02016-6

Leave a Reply